In order to electroplate spoon with silver, the arrangement of the electrolytic cell is?
- A. the anode is a silver rod and the cathode is the spoon
- B. the anode is the spoon and the cathode is a silver rod
- C. the electrolyte is silver trioxonitrate(v)( solution and the cathode is a silver rod.
- D. the electrolyte is silver trioxonitrate(v) solution and the anode is the spoon
A colored gas that is known to be poisonous and can readily damage the mucous lining of the lungs is?
- A. hydrogen sulphide
- B. carbon(ii)oxide
- C. chlorine
- D. sulphur(iv)oxide
An organic functional group which can likely decolorize ammoniacal silver nitrate is?
- A. alkene
- B. alkane
- C. alkyne
- D. alkanol
Zn + 2HCL → ZnCl\(_2\) + H\(_2\)
What happens to zinc in the above reaction?
- A. oxidized
- B. a reactant
- C. reduced
- D. a metal
N\(_2\)O\(_4\) ⇔ 2NO\(_2\) (Δ = -ve)
From the reaction above, which of these conditions would produce the highest equilibrium yield for N\(_2\)O\(_4\)?
- A. Low temperature and high pressure
- B. Low temperature and low pressure
- C. high temperature and low pressure
- D. high temperature and high pressure
An organic compound with fishy smell is likely to have a general formula?
- A. RCONHR\(^1\)
- B. RCONH\(_2\)
- C. RNH\(_2\)
- D. RCOR\(^1\)
Using the metal activity series, the metal that can liberate hydrogen gas from steam is?
- A. iron
- B. copper
- C. tin
- D. lead
Addition of charcoal to the filter bed of sand during water treatment for township supply is to?
- A. prevent goiter
- B. prevent tooth decay
- C. remove odour
- D. kill germs
On the basis of the electrochemical series, which of these ions will show the greater tendency to be discharged at the cathode in an electrolytic cell
- A. cu\(^{2+}\)
- B. sn\(^{2+}\)
- C. fe\(^{2+}\)
- D. zn\(^{2+}\)
A chemical widely used as a fertilizer is?
- A. galena
- B. bauxite
- C. emerald
- D. nitrochalk
Addition of sodium chloride to water to form a solution would lead to?
- A. increase in freezing point and increase the boiling point
- B. increase in freezing point and decrease the boiling point
- C. decrease in freezing point and decrease the boiling point
- D. decrease in freezing point and increase the boiling point
An organic compound which liberate carbon(iv)oxide from trioxocarbonate(iv) solution is likely to be?
- A. C\(_2\)H\(_5\)OH
- B. C\(_3\)H\(_4\)
- C. C\(_6\)H\(_6\)
- D. CH\(_3\)COOH
The sulphide that is commonly used in coating electric fluorescent tubes is?
- A. iron(ii)Sulphide
- B. tin(ii)sulphide
- C. Zinc Sulphide
- D. lead(iv) Sulphide
How many neutrons are present in atom with mass number and atomic number 37 and 17 respectively?
- A. 18
- B. 20
- C. 37
- D. 17
The following non-metal form acidic oxides with oxygen except?
- A. phosphorus
- B. sulphur
- C. carbon
- D. chlorine
In the preparation of salts, the method employed will depend on the?
- A. composition
- B. dissociating ability
- C. stability to heat
- D. precipitating ability
SO\(_2\) + O\(_2\) → 2SO\(_3\)
In the reaction above, the most suitable catalyst is?
- A. chromium(vi)oxide
- B. iron(iii)oxide
- C. copper(i)oxide
- D. vanadium(v)oxide
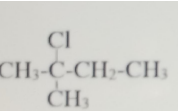
The IUPAC nomenclature of the structure is?
- A. 3-chloro-3-methylbutane
- B. 2,2,-dichloro-3-methylbutane
- C. 3-methylchlorobutane
- D. 2-chloro-2-methylbutane
The sub-atomic particles located in the nucleus of an atom are?
- A. neutron and proton
- B. proton and electron
- C. proton and ions
- D. neutron and electron


