Water can be obtained as the only product during the?
- A. combustion of hydrocarbons
- B. neutraliization of an acid by base
- C. combustion of hydrogen
- D. electrolysis of brine
When sodium trioxocarbonate (IV) decahydrate loses its water of crystallization to the atmosphere, the process is?
- A. deliquescence
- B. efflorescence
- C. hygroscopic
- D. effervesecence
The mixture of gases used in a photographer’s flash tube is?
- A. argon and krypton
- B. krypton and xenon
- C. helium and argon
- D. argon and xenon
The type of bonding in \({Cu(NH_3)_4}^2\)+ is?
- A. coordinate covalent
- B. electrovalent
- C. metallic
- D. covalent
Which quantum number divides shells into orbitals?
- A. principal
- B. Arzimuthal
- C. Magnetic
- D. Spin
Cathode rays causes an object placed behind a perforated anode to cast a shadow on the screen. This observation shows that the rays?
- A. are positively charged
- B. are negatively charged
- C. have mass
- D. travel in straight lines
If an atom is represented as \( _{11}^{23}X \), which of the following deduction is correct?
- A. it contains 12 protons
- B. it forms a covalent chloride
- C. its atomic number is 23
- D. it is an alkali metal
The basic assumption in the kinetic theory of gases that the collisions of the gaseous molecules are perfectly elastic implies that the?
- A. forces of attraction and repulsion are in equilibrium
- B. gaseous molecules can occupy any available space
- C. gaseous molecules will continue their motion indefinitely
- D. gases can be compressed
A gas exerts pressure on its container because?
- A. the molecules of a gas collide with the walls of the container
- B. some of the molecules are moving faster than others
- C. of the collisions of the molecules with each other
- D. of the mass of the molecules of the gas
0.0075 mole of calcium trioxocarbonate (IV) is added to 0.015 mole of a solution of hydrochloric acid. The volume of gas evolved at s.t.p is?
[Molar volume of a gas at s.t.p = 22.4 dm3]
- A. 224cm3
- B. 168cm3
- C. 112cm3
- D. 100cm3
The percentage of water of crystallization in ZnSO\(_4\).7H\(_2\)O is?
[Zn = 65, S = 32, O = 16, H = 1]
- A. 33%
- B. 44%
- C. 55%
- D. 87%
Which of the following is a physical change?
- A. Freezing ice cream
- B. dissolving calcium in water
- C. burning kerosene
- D. Exposing white phosphorus to air
A mixture of sugar and sulphur can be separated by?
- A. dissolution in water, evapouration and filtration
- B. filtration, evaporation and dissolution in water
- C. dissolution in water, filtration and evaporation
- D. evaporation, dissolution in water and filtration
Detergents are manufactured with straight hydrocarbon chains so as to make them
- A. soluble
- B. biodegradable
- C. cheaper
- D. foamy
Polyvinyl chloride is used in the production of
- A. glass
- B. alloy
- C. pipes
- D. ceramics
The product obtained when a mixture of benzene vapour and hydrogen are passed over nickel catalyst at 180ºC is
- A. cyclohexane
- B. cyclopentane
- C. n- hexane
- D. n - pentane
Reduction of alkanones with LialH4 produces
- A. primary alkanols
- B. secondary alkanols
- C. tertiary alkanols
- D. polyhydric alkanols
Reduction of nitroalkanes, nitrites and amides is a route for the preparation of
- A. amines
- B. alkenes
- C. polymers
- D. detergents
A red precipitate of copper (I) carbide is formed when ammonium solution of copper (I) chloride is introduced into
- A. CH2 = CH - CH2 - CH3
- B. CH3 CH2 - C ≡ CH
- C. CH3 CH2 CH2 CH3
- D. CH3 - C C - CH3
A hydrocarbon X with a molar mass of 26 consists of 92.3% carbon. What is its molecular formular?
- A. C2H2
- B. C3H3
- C. C4H4
- D. C5H5
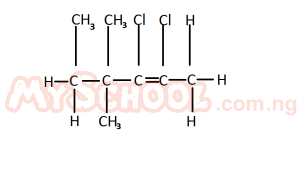
The IUPAC nomenclature for the structure above is
- A. 2, 3 - dichloro - 4, 4, 5 - trimethyl pent - 2 - ene
- B. 4, 5 - dichloro - 2, 3 - dimethyl hex - 2 - ene
- C. 2, 3 - dichloro - 4, 4 - dimethyl hex - 2- ene
- D. 2, 3 dichloro - 2, 2 - dimethyl hex - 2 - ene


