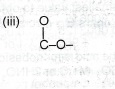(a) Write the structural formula of:
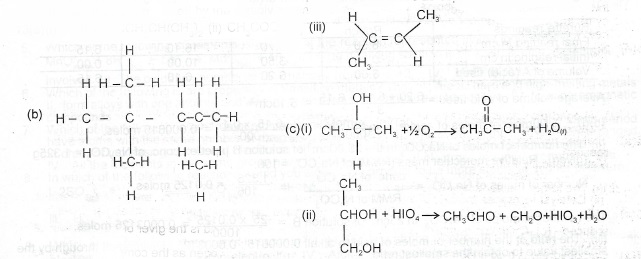
(i) 2, 2, 4 – trimethylpentane, (ii) ethylmethanoate, (iii) trans 2, 3 – dimethylbut – 2-ene.
(b) Write the structure of the straight-chain compound that is isomeric with 2,2,4 – trimethylpentane.
(c) Write chemical equations to illustrate the oxidation of: (i) a secondary alkanol (ii) a dihydric alkanol.
(d) When-Crushed cassava was warmed with dilute hydrochloric acid, a sweet-tasting compound, D was obtained. When compcund D was treated with the enzyme, zymase and the mixture distilled a clear and colourleCsliduid, E was obtained. When liquid E was warmed with eth anoic acid i n the presence of a few drops of concentrated tetraoxosulphate (V1) acid, a compound F, with fruity smell was obtained
. (i)To what class of compounds does D belong?
(ii) Name E and F
(iii) Write the fun-ctional group in F
(iv) Write the equation for the reaction between E and ethanoic acid in the presence of concentrated tetraoxosulphate (VI) acid.
(v) Name the type of reaction that takes place between E and ethanoic acid.
(e) Arrange the followingcompounds in their correct order of increasing boiling points: CH\(_3\)CH\(_2\)CH\(_2\)OH, CH\(_3\)CH\(_2\)CH\(_2\)CH\(_2\)CH\(_2\)CH\(_3\) and CH\(_2\)CH\(_2\)CH\(_2\)CH\(_3\); Explain the order.
Explanation
(a)(i) (CH\(_3\))\(_3\)CCH\(_2\)CH(CH\(_3\))\(_2\)
(ii) CH\(_3\)COOCH\(_3\)
(d)(i) Carbohydrates
(ii) E and F are respectively ethanol and ethylethanoate