(a) A mixture consist of sodium chloride, ammonium chloride and lead (II) tetraoxosulphate (VI). Describe how you would obtain each component in a pure state.
(b) You are required to determine the concentration of a solution of tetraoxosulphate (VI) acid by titrating it against sodium trioxocarbonate (IV) solution of known concentration.
(i) Name the indicator you would use
(ii) List two precautions you would take to ensure accurate readins
(iii) State the colour of the sodium trioxocarbonate (IV) solution after adding the named indicator and then at the end point.
Credit will be given for strict adherence to instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
Carry out the following exercises on sample F. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a) Put all of F into a beaker and add about 10cm\(^3) of water. Stir the mixture and filter. Keep both the residue and the filtrate.
(b)(i) Test the filtrate with litmus paper.
(ii) Add a few drops of the fehling’s solution provided to about 2cm\(^3\) of the filtrate in a test tube. Boil the mixture.
(c) Put the residue from (a) above into a test tube and add about 5cm\(3\) of hydrochloric acid. Identify the gas evolved.
(d) To about 2cm\(^3\) of the clear solution from (c) above add dilute sodium into hydroxide solution dropwise and then in excess.
(e) From your results deduce what F is and its constituent
D is 2.00M ethanoic acid. E is 2.00M potassium hydroxide solution.
(a) Using a 50cm\(^3\) measuring cylinder, measure 50cm\(^3\) of D and transfer the solution into plastic cup.Record the temperature T\(_1\) of the solution. Rinse the cylinder distilled water and allow to dry.
(b) Using the dry measuring cylinder from (a) above, measure 50cm of E. Record the temperature, T\(_2\) of the solution.
(c) Find the average temperature T\(_3\) of the two solutions and record the value.
(d) Pour the measured quantity of solution E quickly from the measuring cylinder into the plastic cup containing solution D. Stir the mixture with the thermometer. Record the highest temperature T\(_4\) attained.
(e)(i) Find the rise in temperature (T\(_4\) – T\(_3\) ad record the value
(ii) Calculate the mass of the reaction mixture, given that during the reaction 1cm\(^3\) of the mixture weighs 1g
(f) From your results in (a) to (e) above, calculate the;
(i) Heat evolved during the reaction, giving that the specific heat capacity of water is 4.2Jg\(^{-1}\)C \(^{-1}\) and using the formula; heat evolved = mass x specific heat capacity x rise in temperature
(ii) Heat of neutralization of one mole of ethanoic acid by potassium hydroxide
(g) List two sources of error in the method used for determining the heat of neutrailzation and suggest how their effect can be minimized.
(a) Explain the differences in the reactions of zinc with dilute trioxonitrate (V) acid and zinc with dilute hydrochloric acid.
(b) Write equations to illustrate how ammonia gas can be converted into trioxonitrate (V) acid.
(c) Calculate the mass of sodium trioxonitrate (V) produced when 30.0g of pure sodium hydroxide reacts with 100cm\(^3\) of 1.00 M trioxonitrate (V) acid. (H =1, N = 14, 0 = 16, Na = 23)
(d) Write the equations for the decomposition by heat of:
(i) sodium trioxonitrate(V);
(ii) copper (II) trioxonitrate (V);
(iii) mercury (II) trioxonitrate (V);
(a) Distinguish between a conductor and an electrolyte
(b)(i) State Faraday’s first law of electrolysis
(ii) Describe how you would investigate Faraday’s law of electrolysis, using copper (II) tetraoxosulphate (VI) solution and copper electrodes.
(c) 0.222g of a divalent metal is deposited when a current of 0.45 ampere is passed through a solution of its salt for 25 minutes using appropriate electrodes. Calculate the relative atomic mass of the metal. 1F = 96500C mol\(^{-1}\)
`(d) State two applications of electrolysis.
(a) List three characteristics of a homologous series
(b) Give one example of;
(i) alkanes; (ii) alkynes.
(c) A hydrocarbon contains 7.7% by mass of hydrogen and 92.3% by mass of carbon. The relative molar mass of the compound is 78.
(i) Derive the empirical formula of the compound and hence its molecular formula.
(ii) Name the hydrocarbon and write its structural formula. (H=1, C=12)
(d) Two hydrocarbons, X and Y were treated separated with acidified potassium tetraoxomanganate (VII) solution. X decolorized the solution and Y did not. Which of X and Y will undergo
(i) substitution reaction only,
(ii) both addition and substitution reactions.
(iii) polymerization?
(e) If ethanol is to be converted into ethanoic acid
(i) What are the conditions required?
(ii) name the type of reaction that will be involved and write the equation
(a) State Le Chatelier’s principle
(b) Use Le Chetelier’s principle to deduce the conditions that favour a high yield of ammonia in the Haber process
(c) Give the chemical test for ammonia.
(d) State what would be observed when aqueous ammonia solution is added to:
(i) zinc chloride solution, (ii) copper (II) tetraoxosulohate (V) solution
(e) Explain why the H — N — H bond angle in ammonia is less than that of H — C — H in methane
(f) Give two uses of ammonia.
(a) Name the components of
(i) producer gas; (ii) water gas;
(b) Give the reason why water is a better fuel than producer gas.
Consider the compounds represented as A and B below:
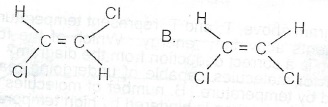
(a) What is the celationship between A and B? (
b) Name A and B.
(c) Will the chemical properties of A and B be the same? Give one reason for your answer.
(a) The half-life of 56/25 Mn is 9.3 x 10\(^3\)S. What does the statement mean?
(b) Give the three types of radiation that are usually emitted by radioactive substances.
(a) Name the gas evolved when dilute hydrochloric acid is added to each of the following solids and the mixture is warmed
(i) sodium trioxocarbonate (IV);
(ii) potassium trioxosulphate (IV);
(iii) iron (II) sulphide.
(b) Name the reagent(s) that you would use to identify the gas in (a)(ii) above.
Name the type of chemical process involved in the production of
(a) polythene from ethene
(b) ethene from kerosene fraction of petroleum.
(c) soap from vegetable oils
(d) margarine from vegetable oils
(a) Write the formula of the oxide of nitrogen in which nitrogen has oxidation number of (i) +1;
(ii) +2;
(iii) +3;
(iv) +4;
(b) State which of the oxides in (a) above is/are: (i) acidic (ii) neutral.
(a) Give two characteristic features of boiling
(b) What will be the effect of the following on the boiling point of water:
(i) addition of crystals of sodium chloride,
(ii) reduction of the atmospheric pressure?
(c) State two ways in which boiling differs from evaporation.
(a) Give one physical property of
(i) diamond;
(ii) graphite
(b) Give two uses of diamond
(c) evidence that shows that both graphite and diamond are allotropes of carbon.
(a) When is a sample of water said to be hard?
(b) State one difference between temporary and permanent hardness of water
(c) Give one method of removing hardness completely from water
(d) Name two local is used for the production of soap.
(a) Write the chemical equation for the formation of named alkanoate.
(b)(i) What are the monomers of protein called?
(ii) Write the two functional groups present in the monomers named in (b)(i) above
(iii) State the type of reaction that leads to the formation of proteins from their monomers.
What is the voltage of the cell represented at Zn(s) Zn 2+(aq) /Cu2+(aq) /Cu(s) given that for Cu 2+(aq)/ Cu (s) Eo = 0.337V and for Zn2+(aq) /Zn(s)’ E o = -0.763V?
- A. +0.426V
- B. -0.426V
- C. -0.337V
- D. -1.10V
- E. +1.10V
The oxidation number of chlorine is + 1 in
- A. KCIO 3
- B. CI 2O 7
- C. ZnCI2
- D. HCI
- E. NaCIO
50cm 3 of a saturated solution of potassium trioxonitrate (V)at 40°C contains 5.05g of the salt. What is the solubility of potassium trioxonitrate (V) at 40°C? (KNO3 = 101)
- A. 1.0 mol dm-3
- B. 1.5 mol dm-3
- C. 2.0 mol dm-3
- D. 2.5mol dm-3
- E. 5.0mol dm-3
Which of the following is a condition for a spontaneous reaction?
- A. ∆H -T is zero
- B. ∆H-T∆ S is positive
- C. ∆H-T∆ S is negative
- D. ∆S is zero
- E. ∆S is positive


