A hydrocarbon X which decolorizes bromine water but has no action on ammoniacal silver trioxonitrate (V) solution was found to have a molar mass of 58 g mol\(^{-1}\)
(a) Deduce the molecular formula of X. (H = 1, C = 12)
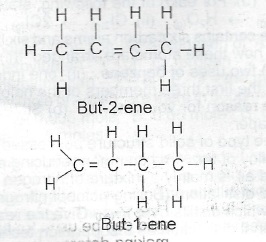
(b) Write the structures of two isomers of X.
Explanation
(a) The test carried out shows the hydrocarbon x is an alkene. The smallest number of the family is ethene (C\(_2H_4\))
If (\(C+2H_4)_x = 56\)
i.e. (28)x = 56
x = \(\frac{56}{28}\) = 2
2(\(C_2H_4\)) = C\(_4\)H\(_8\)
(b)