(a)(i) Define oxidation in terms of electron transfer.
(ii) Write balanced equations for the half reactions for the following changes in acidic solution: Mn0\(^-_4\) + Fe\(^{2+}\) —> Mn\(^{2+}\) + Fe\(^{3+}\)
(b)(i) Distinguish between an electrolytic celI and an electrochemical cell.
(ii) Sketch a cell for the electrolysis of molten magnesium chloride. Lable the anode and the cathode and indicate the direction of electron flow. Give the electrode reactions.
(iii) Give one reason why a platinum anode is not suitable for the eloctrolysis in (b)(i) above.
(c) Calculate the mass of lead that would be deposited from a solution of lead (II) trioxonitrate by the same quantity of electrically depositing 1.35g of copper. (Cu = 63.5, Pb = 207)
Explanation
(a)(i) Oxidation is the process of electron loss. E.g \(Fe_{(g)} \to Fe^{2+}_{(aq)} + 2e^-\)
(ii) \(Fe^{2+}_{(aq)} + e^-\) Oxidation half reaction
\(MnO^- _{4(aq)} + 8H^+_{(aq)} + 8e^- \to Mn^{2+}_{(aq)} + 4H_2O\)
Reduction half rxn.
(b)(i) Difference between electrolytic cell and electrochemical cell.
|
|
Electrochemical cell |
|
Electrica energy is use to ring about chemical energy. Cathode electrode is negatively charged. |
Chemical energy is used to bring about electrical energy. The anode is the negatively charged electrode. |
(ii)
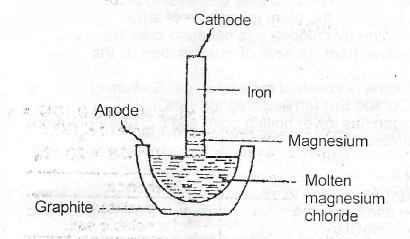
(iii) The platinum anode is attced by chlorine gas.
(c) If 63.5g Cu is equivalent to \(\frac{207}{63.5}\)
1.35g Cu will be equivalent to \(\frac{207}{63.5} \times \frac{1.35}{1}\) = 4.4g Pb

