All your burette readings (initial and final), as well as the size of your pipette, must be recorded but on no account of experimental procedure is required. All calculations must be done in your answer book.
F is a solution, O a dibasic acid H\(_2\)X. G is a solution containing 1.00g of sodium hydroxide in 250cm\(^3\) of solution.
(a) Put F in the burette and titrate with 20cm\(^3\) or 25cm\(^3\) portion of G using methyl orange as indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of F used
(b) From your results and the information provided, calculate the;
(i) concentration of G in mol. dm\(^3\)
(ii) concentration of F in mol. dm\(^{-3}\)
(iii) molar mass of the acid H\(_2\)X, given that 100cm\(^3\) of solution F contained 0.4850 the acid.
The equation for the reaction is H\(_2\)X\(_{(aq)}\) + 2NaOH\(_{(aq)}\) \(\to\) Na\(_2\)X\(_{(aq)}\) + 2H\(_2\)O [H = 1; O = 16; Na = 23]
Credit will be given for strict adherence to instructions, for observations precisely recorded and for accurate inferences. All tests observations and inferences must be clearly entered in your answer book. in ink, at the time they are made.
H is a mixture of an element and an organic compound. Carry out the following exercises on H. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test
(a) Put all of H in a beaker and add about 10cm\(^3\) of distilled water. Stir the mixture thoroughly and filter Keep both the filtrate and the residue. Test the filtrate with litmus paper.
(b) Divide the filtrate into two portions
(i) To the first portion add two to three drops of acidified potassium tetraoxomanganate (VII) and warm
(ii) To the second portion. add a few drops of a saturated solution of sodium hydrogentrioxocarbonate (IV)
(c)(i) Put all the residue in a test tube and add 5cm\(^3\) of dilute hydrochloric acid
(ii) To the resulting solution from (C)(i) above add aqueous sodium hydroxide in drops until it is in excess
(iii) From your inferences in (c)(i) and (c)(i) state what would be observed if 5cm\(^3\) of dilute trioxonitrate (V) acid were added to a portion of the residue and the mixture was warmed.
(a)(i) Define a standard solution
(ii) Give the reason why a standard solution of sodium hydroxide cannot be made by weighing out accurately a given mass of the solid and making it up to the required volume of solution
(b) Give two ways by which a solid solute can be made to dissolve more quickly in a liquid Solvent
(i) Draw a labelled diagram of the apparatus used for drying solids in the laboratory
(c) State what would be observed if a fresh precipitate of silver chloride was;
(i) Exposed to light.
(ii) Shaken will excess aqueous ammonia
(a)(i) List two uses of aluminium and state how each use is related to the properties of the element.
(ii) State the reason why aluminium oxide is said to be amphoteric.
(b) Calcium is extracted by the electrolysis of fused calcium chloride containing about one-sixth of its mass of calcium fluoride.
(i) Sketch and label the cell used for the extraction.
(ii) Write equations for the reactions at the electrodes.
(iii) State the role of the calcium fluoride in the extraction.
(c) W, X, Y and Z represent four metals which have the following properties: W does not react with cold water but it liberates hydrogen from steam; X is one of the products formed when its trioxonitrate (V) decomposes on strong heating; Z forms the oxide when heated in air and it displaces W from an aqueous solutions of a salt of W; Y tarnishes rapidly exposure and reacts vigorously with cold water. Use the information provided to deduce the order of reactivity of the metals.
(a)(i) State Graham’s law of diffusion.
(ii) Consider the reaction represented by the following equation:
N\(_2\)O\(_{4(g)}\) 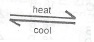 2NO\(_{2(g)}\)
2NO\(_{2(g)}\)
Night yellow dark brown
State what would happen to the vapour density of N\(_2\)O\(_4\) as the temperature of the system is increased. If the system is cooled, would the gases become lighter or darker in colour? Explain your answer in each case.
(b) Explain the following observations:
(i) an inflated balloon that was left in the sun. burst after some time;
(ii) a pure sample of a liquid did not have a constant boiling point at the top and at the base of a high mountain
(c)(i) List two gaseous reducing agents
(ii) Write one equation each to illustrate the reducing property of the gases you listed in (c)(i) above.
(a) Give the products of the following reactions:
(i) hydrolysis of simple proteins.
(ii) alkaline hydrolysis of fats and oils.
(b) A combustion tube was packed with small pieces of broken clay pot and the tube maintained at a temperature of 750K. When the vapour of decane was passed into the tube, the main products included a gaseous hydrocarbon X.
(i) Name the process involved in the reaction. Give its industrial application.
(ii) State the function of the pieces of broken pot in the experiment.
(iii) Give one chemical test to distinguish between X and methane.
(iv) Draw a labelled diagram for the laboratory preparation of X.
(c)(i) State what would be observed if a piece of sodium was added to 10cm\(^3\) of propanol in a beaker. Write an equation for the reaction.
(ii) Give the main product formed when excess acidified potassium heptaoxodichromate (VI) reacts with each of the following: propan-1- ol: propan – 2 -of; State the type of process involved in the reactions.
(a)(i) State three differences between electrovalent compound and covalent compound.
(ii) Name the type of chemical bonding involved in the formation of ammonium ion from ammonia.
(b)(i) Name the quantum numbers which define an electron within an atom.
(ii) State the orbital in which the fifth electron of an atom is most likely to be found. Sketch the shape of the orbital.
(iii) State the period and the group to which the element boron belongs in the Periodic Table.
(c)(i) What is meant by the entropy of a chemical system?
(ii) Calculate the free energy change for a given reaction at 300 K using the following data obtained for the reaction: \(\Delta\) = -710KJ mol\(^{-1}\); \(\Delta\)S = 0.15 KJ mol\(^{-1}\)K\(^{-1}\)
(iii) From your evaluation in (c)(i) above, state whether the reaction is spontaneous or not at the given temperature. Give reason for your answer.
(a) Name: (i) one structural isomer of glucose.
(ii) the process by which starch is converted to glucose.
(b) The open-chain structure of glucose is shown below.
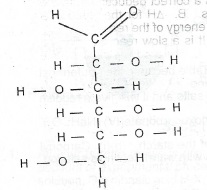
(a) State the functional groups present in the structure.
(ii) Which of the functional groups would react with warm Fehling’s solution?
(a) What is meant by the term acid salt? Give one example.
(b) State the reason why an all-glass apparatus must be used for the laboratory preparation of concentrated trioxonitrate (V) acid.
(a) Name one metal in each case Which:
(i) exists as a liquid at room temperature,
(ii) can be found in nature in the uncombined state
(b) X and Y are 0.5 mol. dm\(^{-3}\) freshly prepared aqueous solutions of two salts of iron
(II) With excess sodium hydroxide solution, X gave a dirty green precipitate which was not obtained in the case of Y.
(a) Three elements A, B and C have atomic numbers 8, 11 and 12 respectively.
(i) Write the formula of the compound formed by the chemical combination of A and B.
(ii) State which of the three elements belong(s) to the s-block of the Periodic Table. Give reason for your answer:
(b) List the component elements of bleaching powder.
(a) Give one example of naturally-occurring acids
(b) If 0.5 mole of a mono-alkanoic acid weighs 44g, determine the molecular formula and the name of the acid. (H = 1, C = 12, O = 16)
Magnesium ribbon reacts with dilute hydrochloric acid at room temperature.
(a) State three ways by which the reaction can be made to proceed faster
(b) Write an equation for the reaction.
(a) Give the electrolyte of a named secondary electrochemical cell.
(b) Consider the cell represented as shown below: \(Cu_{(s)}Cu^{2+}_{(aq)}/Zn^{2+}_{(aq)}/Zn{(s)}\)
(i) What does the vertical double stroke represent?
(ii) Which of the metals forms the cathode?
(iii) Name the electrode to which each half-cell should be coupled if the oxidation potential of the half-cells are to be determined.
(a) Name the crystalline allotrope of sulphur that is stable at room temperature.
(b)(i) Give one example of a fuel that contains significant amount of sulphur as an impurity.
(ii) State one environmental disadvantage of using a fuel that has high sulphur content
(a) State the main ore of tin.
(b) Write an equation for the reaction involved in the smelting of purified tin ore
(c) List two alloys of tin
(a) State the method of collecting gases which are denser than air.
(b) Name two gases that can be used to perform the fountain experiment in the laboratory. State the physical property which makes it suitable for the experiment.
Which of the following accounts for the difference in the mode of conduction of electricity by metals and aqueous salt solutions?
- A. Electrons are present in metals but not in salt solutions
- B. Metals are conductors while salts are electrolytes
- C. Electricity is carried by mobile elrctrons in metals but by ions is aqueous salt solution
- D. salts ionize in aqueous solutions while metals do not
- E. Metals are reducing agents while salts are not
A dye is suspected to have contaminated a lollipop. Which of the following is the best method by which the contaminant may be isolated?
- A. Fractional distillational
- B. Recrystallization
- C. Filtration
- D. Paper chromatography
- E. Evaporation
What is the value of n in the following equation? XO-4 +8H + + ne- → X2 + + 4H2O
- A. 2
- B. 3
- C. 4
- D. 5
- E. 6
Which of the following groups contains entirely linear molecules?
- A. H 2NH3O 2
- B. CO2NH 3 2
- C. H 2CH4N2
- D. CO2H2N2
- E. CH 4O 2CO 2


