A solution containing 0.095 mol. dm\(^{-3}\) of trioxonitrate (V) acid. Solution B contains 13.50g of X\(_2\)CO\(_3\).10H\(_2\)O per dm\(^3\)
(a) Put A in the burette and titrate with 20cm\(^3\) or 25cm\(^3\) portions of B using methyl orange as an indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of A used.
(b) From your results and the information provided, calculate the;
(i) Concentration of B in mol. dm\(^3\)
(ii) molar mass of X\(_2\)CO\(_3\).10H\(_2\)O
(iii) percentage by mass of X in X\(_2\)CO\(_3\).10H\(_3\)O. The equation for the reaction is X\(_2\)CO\(_3\) + 2HNO\(_3{(aq)}\) \(\to\) 2XNO\(_{3(aq)}\) + CO\(_{2(g)}\) + 11H\(_2\)O\(_{(l)}\) [H = 1, C = 12, O = 16]
(c) Give the reason for the following:
(i) using just a small quantity of the indicator during acid-base titrations.
(ii) obtaining at least two sets of readings for titration experiment.
You are provided with two aqueous solutions labelled C and D. Carry out the following exercises on them. Record your observations and identify any gases evolved. State the condition you draw from the result of each test.
(a)(i) Mix C and D in a beaker thoroughly.
(ii) Filter the mixture. Keep both the residue and the filtrate.
(b)(i) To about 5cm\(^3\) of the filtrate, add barium chloride solution, followed by excess dilute hydrochloric acid in a boiling tube. Divide the resulting solution into two portions.
(c)(i) To the first portion of the solution from (b)(ii) above, add sodium hydroxide solution in excess.
(ii) To the second portion of the solution from (b)(ii) above, add aqueous ammonia in drops until it is in excess.
(a) State;
(i) One advantage:
(ii) One disadvantage of evaporation of salt solutions to dryness over crystallization.
(b)(i) List two normal salts which when dissolved in pure water are acidic to litmus
(ii) Mention the phenomenon that accounts for the behaviour of the salts in (b)(i) above.
(c)(i) Outline a suitable laboratory method for separating a mixture of glucose and starch.
(i) Give two chemical tests that would enable you to identify three solids suspected to be glucose, sucrose and starch.
(a)(i) List four characteristic properties of transition metals
(ii) Name two metals that can be extracted from their ore by electrolysis.
(b)(i) Determine the oxidation number of chromium in Cr\(_2\)O\(^{2-}_{7}\)
(ii) State the colour observed on adding a few drops of dilute tetraoxosulphate (VI) acid to the system representedby the following equation: Cr\(_2\)O\(^{2-}_{7(aq)}\) + H\(_2O_{(l)}\) \(\rightleftharpoons\) 2CrO\(^{2-}_{4(aq)}\) + 2H\(^+_{(aq)}\). Explain your answer.
(c)(i) State and explain what would be observed if hydrogen sulphide gas were bubbled into acidified K\(_2\)Cr\(_2\)O\(_7\). Write an equation for the reaction.
(ii) What precaution should be taken to avoid excessive exposure to hydrogen sulphide gas while it is being generated in the laboratory?
(a)(i) What is meant by the rate of a chemical reaction?
(ii) Explain in terms of the vision theory, the effect of temperature increase on reaction rate.
(b) When hydrogen peroxide is exposed to air, it decomposes
(i) Write an equation for the reaction.
(ii) Outline an experiment to illustrate that effect of a named catalyst on the rate of decomposition.
(iii) Sketch an energy profile diagram to show the effect of the catalyst on the reaction rate, given that the reaction is exothermic.
(c)(i) Explain why enthalpy data alone cannot be used to predict whether a reaction can occur spontaneously or not.
(a)(i) Define the term polymerization.
(ii) List the three conditions required for the polymerization on of ethene.
(iii) State the property which is common to compounds that can be easily polymerized
(b) Write appropriate equations to show how the following can be obtained from propan-1-ol in the labouratory
(i) propene;
(ii) propylmethanoate. State the type of reaction involved in each case.
(c)(i) A compound contains 40.0% carbon, 6.7% hydrogen and 53.3% oxygen. Determine its molecular formula if its molar mass is 180 (H = 1, C = 12, O = 16)
(ii) Explain why ethanoic acid boils at a much higher temperature than butane even though their molar masses almost equal.
(a)(i) Distinguish between a strong acid and a concentrated acid.
(ii) What is meant by amphoteric oxide? Give one example.
(b)(i) Describe the manufacture of tetraoxosulphate (VI) acid by contact process.
(ii) Write one equation each to show the action of tetraoxosulphate (VI) acid respectively as a dehydrating agent and as an oxidizing agent.
(iii) Give the reason why tetraoxosulphate classified as a heavy chemical.
The compound whose formula is written below is a major component of a soft fatty substance:
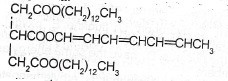
(a) State the change that would be observed in the physical state of the fatty substance if hydrogen were bubbled through it for long time in the presence of finely divided nickel at about 180\(^o\)C
(c) Determine the amount (in mole) of hydrogen that would be consumed if one mole of the component reacted completely with hydrogen.
(c) State the product of the reaction of the fatty substance with hot concentrated sodium hydroxide solution
(a) X and Y belong to the same period in the Periodic Table is a group I element while Y belongs to group VII. State which of the elements would
(i) be a good oxidizing agent
(ii) have the smaller atomic volume
(iii) have the higher ionization potential
(b) Explain your answer in (a)(i) above.
State which of the following can exhibit geometric isomerism:
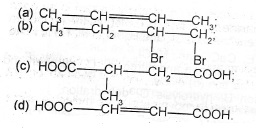
Give reason for your answer
Mention the respective properties of the following allotropes of carbon that account for their uses as indicated:
(a) diamond used for drilling rocks;
(b) diamond used as jewels;
(c) graphite used as electrodes;
(d) graphite used for slowing down neutrons in nuclear reactors;
(e) wood charcoal used in gas masks.
(a) Name the residue obtained on strongly heating the following:
(i) ZnCO\(_3\) in an open crucible;
(ii) CuSO\(_4\), 5H\(_2\)O and then allowing it to cool in a desiccator.
(b) State the colour changes observed on heating and cooling in each case in(a) above.
(a) State the phenomenon illustrated by the:
(i) spreading of the smell of hydrogen sulphide gas in the laboratory;
(ii) existence of atoms of the same element having different mass numbers
(b) The atomic number of an element is 17. It has different atoms containing 18 neutrons and 20 neutrons, with a relative abundance of 75% and 25% respectively. Calculate the relative atomic mass of the element.
(a) If L is the Avogadro constant and E° is standard cell potential, state what X and Y stand for in the following expressions
(i) X = \(\frac{\text{Mass of L molecules of gas or vapour}}{\text{ Mass of L molecules of hydrogen}}\)
(ii) Y = -nFE°
(b) State two differences between a primary cell and a secondary cell.
(a) State one air pollution that causes:
(i) blood poisoning
(ii) acid poisoning
(iii) blackening of the walls of buildings
(b) Mention one major chemical industry in each case which requires the following as raw materials:
(i) petrochemicals;
(ii) cellulose.
(a) Give one disadvantage of:
(i) hard water
(ii) soft water
(b) Explain why the degree of hardness in a sample of clear lime water is higher than in another sample has that been turned milky by carbon (IV) oxide.
Classify each of the following as physical change or a chemical change:
(a) fractional distillation of liquefied air;
(b) cracking of petroleum fractions;
(c) conversion of rhombic sulphur to monoclinic sulphur;
(d) chromatographic separation of chlorophyll.
The alloy used for metal work and plumbing contains
- A. lead and tin
- B. iron and carbon
- C. copper and tin
- D. aluminium and copper
- E. lead and antimony
Zinc displaces copper from an aqueous solution of copper (ll) salt because
- A. copper is a transition element
- B. copper is a moderately reactive metal
- C. Zinc and copper have reducing prperties
- D. zinc is more reactive than copper
- E. zinc reacts with both acids and alkalis
When ammonium chloride is dissolved in water in a test tube, the tube feels colder showing that
- A. the solution is unsaturated
- B. sublimation has ocurred
- C. ammonia gas is evolved
- D. the process is endothermic
- E. condensation has occurred
The product of the reaction between ethanol and excess acidified K2Cr2O2 is
- A. ethanal
- B. ethylethanoate
- C. ethanoic acid
- D. ethyne
- E. ethanedioic acid


