(a) List two differences between solids and liquids.
(b) The graph below is the heating curve for a solid X. Use the graph to answer Questions (i) — (iii) below.
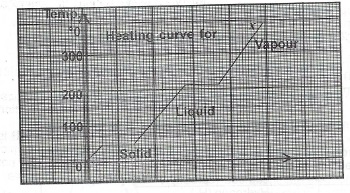
(i) What is the melting point of X?
(ii) If the vapour of X is cooled, at what temperature will it start to condense?
(iii) (I) As X is heated, state what happens to the: I. frequency of collision of molecules of X;
(II) value of the entropy of the system.
Explanation
| Solids | Liquids |
|
1. Have definite 2. Particles are closely packed |
Assume the shape of container Loosely bound particles |
(b)(i) The melting point of x is 60°C
(ii) At 210°C
(iii)(i) The frequency of collision of molecules of x increases
(ii) The value of the entropy of the system also increases.

