(a)(i) List three factors which affect the discharge of ions during electrolysis
(ii) Describe in outline, the purification of copper by electrolysis.
(b) the following data were collected in an experiment on electrolysis:
| Current flowing(amps) | Time of current flow (secs) | Quantity of electricity (coulombs) | Mass of metal M deposited (gram) |
|
0.20 0.20 0.20 0.20 |
900 1800 2700 3600 |
0.06 0.12 0.18 0.24 |
(i) Copy and complete the table above calculating the quantity of electricity passed in each case
(ii) Plot a graph of the mass of M deposited against the quantity of electricity passed.
(iii) From the graph, determine the mass of M that was deposited by the same current passing for 20 minutes.
(iv) From the shape of the graph, which of the laws of electrolysis does the experiment verify?
Explanation
(a)(i) (1) Position of ions in the activity series or electrochemical series
(2) The concentration of ion in the electrolyte
(3) Nature of electrode.
(ii) The impure copper is made up of the anode in electrolyte while strips of pure copper act as cathode. The electrolyte is copper (II) tetraoxosulphate (VI) solution. During electrolysis, the impure copper anode dissolves and copper ions are transferred to the cathode when they are discharged and deposited on the pure copper.
At the anode Cu \(\to\) Cu\(^{2+}\) + 2e\(^+\)
At the cathode Cu\(^{2+}\) + 2e\(^{2+}\) \(\to\) Cu
(b)
| Current flowing(amps) | Time of current flow (secs) | Quantity of electricity (coulombs) | Mass of metal M deposited (gram) |
|
0.20 0.20 0.20 0.20 |
900 1800 2700 3600 |
180 360 540 720 |
0.06 0.12 0.18 0.24 |
(ii)
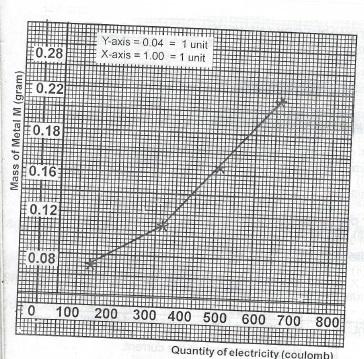
(iii) Quantity of electricity passing for 20 minutes = (20 x 60) Sec. X 0.2 = 240 coulombs. From the graph, mass of M deposited by 240 coulombs is 0.08g
(iv) Faraday's 1st law of electrolysis.

