All your burette readings (initial and final), as well as the size of your pipette, must be recorded but on no account of experimental procedure is required. All calculations must be done in your answer book.
A is 0.50 mol dm\(^{-3}\) hydrochloric acid. B is 0.025 mol dm\(^{-3}\) of a trioxocarbonate (IV) salt.
(a) Put A into the burette and titrate with 20.0cm\(^{-3}\) or 25.0 cm\(^{-3}\) portions of B using methyl orange or screened methyl Orange indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average of A used.
(b) From your results, calculate the mole ratio of acid to trioxocarbonate (IV) in the reaction, expressing your answer as a whole number ratio of one.
(c) Given that B contains 7.2g dm\(^{-3}\) of the hydrated trioxocarbonate (IV) salt, calculate the:
(i) concentration of anhydrous salt in B in g dm\(^{-3}\) [Molar mass of anhydrous salt in B = 106g]
(ii) percentage of water of crystallization in the hydrated salt.
Credit will be given for strict adherence to instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C and D are samples of two simple salts. Carry out the following exercises on them. Record your observations and state the conclusion you draw from the result of each test.
(a)(i) Put half of C in a test tube and add about 5cm\(^3\) of distilled water. Test with litmus.
(ii) Put the rest of C in a test tube and add about 5cm\(^3\) of dilute hydrochloric acid. Identify any gases evolved.
(b)(i) Put half of D in a test tube. Add about 5cm\(^3\) of sodium hydroxide solution and warm. Identify any gases evolved.
(ii) Put the rest of D in a test tube and add about 5cm\(^3\) of distilled water. Mix thoroughly. Add about 2cm\(^3\) of barium chloride solution, followed by dilute hydrochloric acid in excess.
(a) Name one laboratory apparatus/set-up for:
(i) determining the heat of neutralization:
(ii) decomposing water into hydrogen and oxygen.
(b) Outline a suitable procedure for distinguishing between glucose and cellulose using
(i) one physical test apart from tasting.
(ii) one chemical test.
(c) Draw a labelled sketch to illustrate the separation of a mixture by sublimation.
(ii) Give two examples of substances that are sublime.
(d)(i) Mention one salt which produces brown fumes on being heated strongly
(ii) What is the action of the brown fumes mentioned in (i) above on litmus.
(iii) Give one reason why it is not advisable to collect nitrogen by displacement of air [N – 14].
(a)(i) List three characteristic properties of transition metals
(ii) 0.45g of a metal M was deposited when a current of 1.8 amperes was passed for 12.5 minutes through a solution containing M\(^{2+}\). Calculate the relative atomic mass of M. [1 Faraday = 96500 C]
(iii) Give the reason why copper-plated iron corrodes easily when the surface is scratched.
(b)(i) State the law of definite proportions (constant composition).
(ii) Describe in outline, an experimental procedure for determining the proportion of oxygen in a given sample of copper(II) oxide.
(iii) Write an equation to show how copper (II) oxide can be obtained directly from copper (II) trioxonitrate (V)
(a)(i) What is meant by cracking of petroleum fractions?
(ii) Write an equation for the laboratory preparation of ethene from ethanol.
(iii) Give one chemical test to distinguish between ethane and ethene.
(b)(i) Name the class of carbohydrates to which starch and cellulose belong.
(ii) What process is used for isolating ethanol from the other products of fermentation of sugar?
(iii) Name the organic product of the reaction between ethanol and sodium
(iv). Write the structural formula of 2-chloroethanol.
(c) State the reason why:
(i) benzene produces more soot than ethene on burning in excess air;
(ii) ethanoic acid has a higher boiling point than methanoic acid;
(iii) sodium chloride is used during the manufacture of soap.
(d) Give one use of: (i) ethyne (ii) coal (iii) carbon black
(a)(i) State two general methods of preparing soluble salts.
(ii) Mention three pieces of apparatus required for determining the solubility of a salt at a given temperature.
(b) The solubilities of two salts represented as K and L were determined at various temperatures. The results are shown in the table below:
| Temperature (\(^o\)C) | 0 | 20 | 40 | 60 | 80 | 90 |
| Solubility of K (mol. dm\(^{-3}\)) | 0.38 | 0.46 | 0.54 | 0.62 | 0.69 | 0.73 |
| Solubility of L (mol. dm\(^{-3}\)) | 0.12 | 0.34 | 0.64 | 1.08 | 1.64 | 2.00 |
(i) Plot the solubility curves of K and L on the same graph. Use the curves to answer questions (ii) – (iv) below.
(ii) What is the solubility of K at 50°C?
(iii) At what temperature is the solubility of L equal to 1.0mol. dm\(^{-3}\)?
(iv) Over what temperature range is K more soluble that L?
(v) Given that the molar mass of L is 101g, determine whether a solution containing 3.4g of L per 250cm\(^3\) at 20°C is saturated or unsaturated.
(a)(i) List two reactants for the laboratory preparation of ammonia
(ii) State three physical properties of ammonia
(iii) Describe in outline, the manufacture of ammonia by the Haber process.
(b) Write an equation in each case to show the:
(i) reaction between ammonia gas and heated copper (II) oxide
(ii) action of heat on ammonium trioxocarbonate (IV)
(c)(i) Which industrial process is used for convey ammonia to trioxonitrate (V) acid?
(ii) Give the reason why electropositive metals do not generally are off hydrogen with dilute trioxonitrate (V) acid
(d) Give one example in each case, to show how trioxonitrate (V) acid reacts generally with: (i) bases (ii) non-metals.
(a)(i) Define entropy
(ii) What term is used to describe a reaction in which heat is absorbed from the surrounding?
(b) State two conditions that can lead to ineffective collisions during a chemical reaction.
(a) Give one example of a metal which:
(i) can displace hydrogen from cold water
(ii) at red-heat, reacts reversibly with steam
(iii) does not react with dilute hydrochloric acid
(b) When dry hydrogen was passed over lead (II) oxide, a greyish solid J was obtained.
(i) Identify J
(ii) What type of reaction was involved in the formation of J from lead (II) oxide?
(a) Mention three chemical properties of chlorine
(b) What type of reaction is represented by each of the following equations?
(i) Mg\(_{(s)}\) + 2HCI\(_{(aq)}\) –> Mg\(_2\)Cl\(_{2(aq)}\) + H\(_{2(g)}\)
(ii) Ag\(^+_{aq}\) + Cl\(^-\)\(_{(aq)}\) —-> AgCl\(_{(s)}\)
(a) State Gay Lussac’s law of combining volumes
(b) Hydrogen reacts with oxygen according to the following equation;
2H\(_{2(g)}\) + O\(_{2(g)}\) —> 2H\(_2\)O\(_{(g)}\). If 50cm\(^3\) of hydrogen were sparked with 30cm\(^{3}\) of oxygen, calculate the volume of unused oxygen after cooling to the initial temperature and pressure.
(a) List two products obtained when crude oil is refined
(b)(i) What is the general formula for alkanols?
(ii) State the type of reaction involved in the conversion of ethanol to ethanoic acid
(iii) Write an equation to show how ethanoic acid reacts with sodium trioxocarbonate (IV).
Consider the reaction represented by the following Q equation: N\(_2\)O\(_{4(g)}\) \(\rightleftharpoons\) 2NO\(_{2(g)}\) \(\Delta\)H = +57.2KJmol\(^{-1}\)
(a) When is the reaction said to be at equilibrium?
(b) Mention two conditions that can favour the forward reaction
(c) Name the principle involved in (b) above.
(a) State two properties of alkalis
(b)(i) Why is tetraoxosulphate (VI) acid able to produce two types of salt?
(ii) Write an equation for the reactior involved on heating sodium trioxosulphate (IV) with hydrochloric acid.
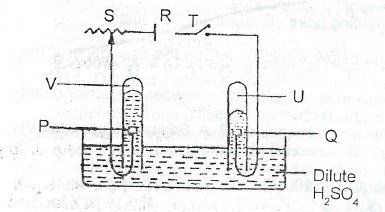
(a) List two industrial uses of concentrated tetraoxosulphate (VI) acid
(b) The diagram below represents the set-up for the electrolysis of dilute tetraoxosulphate (VI) acid. Use it to answer Questions (i) to (iii).
(i) Which letter on the diagram represents the battery?
(ii) Write an equation for the reaction occurring at the cathode
(iii) What product does V represent?
(a)(i) State the two main processes involved in the manufacture of oxygen from air
(ii) Name the type of chemical bonding which exists between oxygen atoms in a molecule of oxygen
(b) What term is used to describe the relationship between oxygen and ozone (O\(_3\))?
(a) List two properties of alpha particles.
(b) X is an element which exists as an isotopic mixture containing 90% of \(^{39}_{19} X\) and 10% of \(^{41}_{19}X\).
(i) How many neutrons are present in the isotope \(^{41}_{19}X\)?
(ii) Calculate the mean relative atomic mass of X.
What is the basicity of the acid in the followed reaction? NaCO + 2CHCOOH → 2CH COONa + H2O + CO2
- A. 1
- B. 2
- C. 3
- D. 4
- E. 8
A mixture of NaCI(s) and CaCO3(s) is best separated by
- A. dissolution followed by filtration
- B. sublimation followed by crystallization
- C. dissolution followed by evaporation
- D. dissolution followed by crystallization
- E. sublimation followed by dissolution
What volume of oxygen at s.t.p would react with carbon to form 4.40g of CO2 according to the following equation? C(s) + O2(g) → CO2(g) [O = 16; C =12; 1 mole of a gas occupies 22.4dm3 at s.t.p]
- A. 0.224dm3
- B. 2.24dm3
- C. 4.40dm3
- D. 4.48dm3
- E. 22.4dm3
Boyle’s law can be expressed mathematically as
- A. V \(\alpha\) T (P constant)
- B. P \(\alpha\) V (T constant)
- C. P \(\alpha\) T
- D. V \(\alpha\) 1 / p(T constant)
- E. V1/T1 = / T2


