(a)(i) State two general methods of preparing soluble salts.
(ii) Mention three pieces of apparatus required for determining the solubility of a salt at a given temperature.
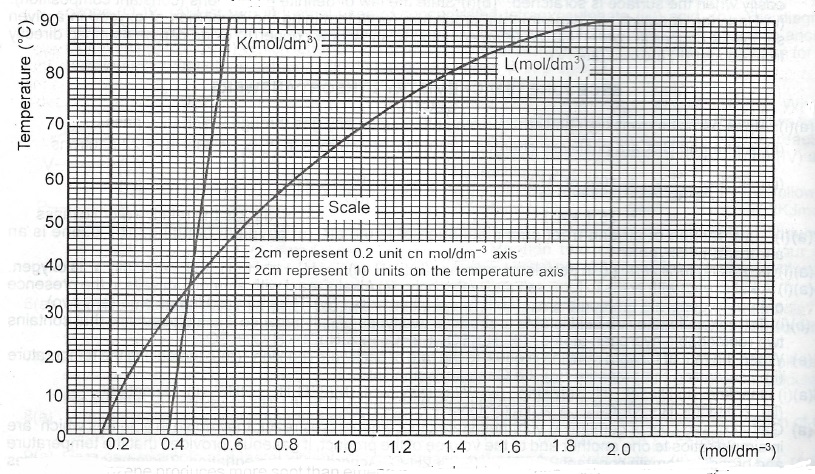
(b) The solubilities of two salts represented as K and L were determined at various temperatures. The results are shown in the table below:
| Temperature (\(^o\)C) | 0 | 20 | 40 | 60 | 80 | 90 |
| Solubility of K (mol. dm\(^{-3}\)) | 0.38 | 0.46 | 0.54 | 0.62 | 0.69 | 0.73 |
| Solubility of L (mol. dm\(^{-3}\)) | 0.12 | 0.34 | 0.64 | 1.08 | 1.64 | 2.00 |
(i) Plot the solubility curves of K and L on the same graph. Use the curves to answer questions (ii) – (iv) below.
(ii) What is the solubility of K at 50°C?
(iii) At what temperature is the solubility of L equal to 1.0mol. dm\(^{-3}\)?
(iv) Over what temperature range is K more soluble that L?
(v) Given that the molar mass of L is 101g, determine whether a solution containing 3.4g of L per 250cm\(^3\) at 20°C is saturated or unsaturated.
Explanation
(a)(i) - Neutralization of a base by an acid
- Direct combination of elements
(ii) Thermometer, water bath and stirrer
(b)(i) The solubility of K at 50\(^o\) equal to 0.58mol/dm\(^3\)
(ii) The solubility of L equals to 1.0mol/dm\(^{-3}\) at 57\(^o\)C
(iii) K is more soluble than L over the temperature range of 0\(^o\) to 31.5\(^o\)C
(iv) Molar mass of L = 101g
3.4gm of L per 250cm\(^3\) \(\to\) 3.4 \(\to\) 250X \(\to\) 1000X = \(\frac{1000}{250}\) x 3.4 = 13.6g/dm\(^3\)
At 20\(^o\)C the molar conc. of L = 0.34
The solubility 101 x 0.34 = 34.34g/dm\(^3\) at 20\(^o\)C. Since the concentration/solubility of 13.6g/dm\(^3\) is lower than the required solubility of 34.34g/dm\(^3\) at 20\(^o\)C, it is therefore not saturated ie. unsaturated