What is the value of x in the following equation? Cr2O72- + 14H+ + xe- → 2Cr3+ + 7H2O
The correct answer is: B
Explanation
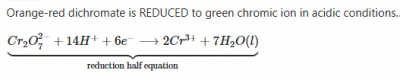
Cr2O72- + 14H+ + xe- → 2Cr3+ + 7H2O
Consider the total ionic substances on the LHS and that on the RHS
LHS= -2 (from Cr2O7 2-) + 14 (from 14H+) +x = -2 +14 + x
RHS= 2 x +3 (from 2Cr3+) = +6
Equate LHS and RHS
-2 + 14 + x = +6 (ensure both values on the LHS and RHS are equal)
+12 + x = +6
x = 6 -12 = -6 (minus because its an electron)
thus, x = 6. Option B is the correct answer (See also image attached)


