(a) If you were provided with anhydrous Na\(_2\)CO\(_3\), spatula and stirrer;
(i) list three other materials you would require to prepare a standard solution of Na\(_2\)CO\(_3\)
(ii) state what you would observe on adding diluted H\(_2\)SO\(_4\) to a portion of the Na\(_2\)CO\(_3\)
(b)(i) Describe briefly one chemical test you would perform to distinguish between zinc ions and aluminium ions in solution.
(ii) Mention one laboratory reagent you would use to;
I. produce ammonia from (NH\(_4\))\(_2\)SO\(_4\)
II. differentiate between precipitates of AgCl and Agl
lll. dehydrate ethanol
(c) Give the reason for each of the following laboratory practices
(i) Aqueous solutions of FeSO\(_4\) are freshly prepared when required for use.
(ii) The first jar of hydrogen collected during its preparation is discarded
Credit will be given for strict adherence to instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is one of the following substances; starch or sucrose or glucose D is a simple salt. Carry out the following exercises on C and D. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a)(i) Add about 5 cm\(^3\) of distilled water to a portion of C in a test tube. Stir thoroughly and test with litmus
(ii) Add about 2cm\(^2\) of Fehling’s solution to the resulting mixture from (a)(i) above the heat.
(b)(i) Heat a portion of D strongly in a test tube
(ii) Put the rest of D in a boiling tube and add about 10 cm\(^3\) of distilled water. Shake the mixture
(iii) Put about 2 cm\(^3\) of the mixture from (b)(ii)) in a test tube. Add aqueous ammonia in drops and then in excess
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but on no account of experiment procedure is required. All calculations must be done in your answer book.
A is mol dm HCI. B is a solution containing 15.0 g dm of a mixture of NaCl and KHCO\(_3\).
(a) Put A burette and titrate it against 20.0cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange as indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is: HCl\(_{aq}\) + KHCO\(_{3(aq)}\) \(\to\) KCl\(_{(aq)}\) +CO\(_{2(g)}\)
(b) From your results and the information provided above, calculate the:
(i) concentration of KHCO\(_3\), in mol dm\(^{-3}\) in B;
(ii) mass of KHCO\(_3\), in g dm\(^{-3}\) in B
(ii) Percentage by mass of KHCO\(_{3}\) in the mixture, [H=1; C = 12; O = 16; K = 39]
(iv) mass of NaCl in the mixture.
(a) Describe briefly a suitable procedure for preparing a pure sample of MgSO\(_4\) starting from MgO.
(b)(i) Mention two sources of water pollution.
(ii) Explain why the sample of air collected in the process of boiling water is richer in oxygen than atmospheric air
(iii) Mention one substance used as coagulant in water treatment plants.
(c)(i) State two physical porperties of chlorine.
(ii) Write an equation to show how chlorine reacts with iron
(iii) Why is Chlorine preferred to sulphur (IV) oxide in the bleaching of cotton
(d) Bleaching powder reacts with dilute HCl according to the reaction below;
CaOCl\(_{2(s)}\) + 2HCI\(_{(aq)}\) -> CaCl\(_{2(aq)}\) + H\(_2\)O\(_{(l)}\) + Cl\(_{2(g)}\)
Calculate the mass of bleaching powder that will produce 400cm\(^3\) of chlorine at 25\(^o\)C and a pressure of 1.20 x 10\(^5\) NM\(^{-2}\). [O = 16.0; Cl = 35.5; Ca = 40.0;1 mole of gas occupies 22.4 dm\(^3\) at s.t.p; standard pressure = 1.01 x 10\(^6\) Nm\(^{-2}\)]
(a) List two substances that can be used in the laboratory to
(i) dry hydrogen;
(ii) remove carbon (IV) oxide from a sample of air;
(iii) convert hot copper (II) oxide to copper;
(iv) prepare zinc chloride by the action of dilute HCI.
(b)(i) Name two alloys which contain lead.
(ii) State and explain what is observed on bubbling H\(_2\)S into a solution of Pb(NO)\(_2\).
(iii) A metal M exists as a silvery white solid at temperatures above 18°C and as a grey solid below 18°C.
I. name the phenomenon exhibited by M.
II. What term is used to describe the temperature given as 18°C in this case?
(c)(i) Write an equation for the action of heat on each of the following compounds:
I. AgNO\(_3\)
II. (NH4)\(_2\)CO\(_3\).
(ii) Copy and complete the table below
|
Metal |
Name of main ore | Method of extraction |
One major use Haematite |
|
— |
Haematite | — |
— |
|
— |
— | Electrolysis of molten oxide |
— |
(a)(i) State three characteristics of a homologous series.
(ii) Give the name and structural formula of the second member of the alkyne series.
(iii) Write an equation to represent the combustion of ethane in excess oxygen.
(b) Name the type of reaction involved in the conversion of ethanol to
(i) ethene;
(ii) ethylethanoate;
(iii) chloroethane;
(iv) ethoxide
(v) ethanoic acid
(c) Consider the following compound.
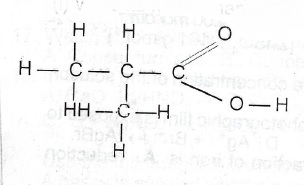
(i) Write its IUPAC name.
(ii) Give its molecular formula and empirical formula
(iii) List the products of its H H 0 reaction with saturated Na\(_2\)CO\(_3\) solution.
(iv) State with reason whether its boiling point will be higher or lower than that of the corresponding alkane.
(d) A vegetable oil X was treated with activated charcoal and then with a gas Y in the presence of a catalyst in order to manufacture
(i) Identify Y.
(ii) State the function of the activated charcoal.
(iii) What is the catalyst used?
(iv) If a sample of X is heated with concentrated sodium hydroxide solution, list the products that will be obtained.
(a)(i) Draw and label a simple cell for the electrolytic purification of copper.
(ii) Write can equation for the reaction at each electrode in (a)(i) above.
(iii) State with reason whether the Daniell cell is an electrolytic cell or an electrochemical cell.
(iv) What is the function of MnO\(_2\) in the Laclanche cell?
(b) Consider the following equation: MnO\(^-_4\) + 8H\(^+\) + xe\(^-\) \(\to\) Mn\(^{2+}\) + yH\(_2\)O. State the
(i) values of x and y;
(ii) oxidation state of Mn in MnO\(^-_4\).
(c)(i) List three factors that affect selective discharge of ions during electrolysis
(ii) State Faraday’s second law of electrolysis.
(iii) A voltameter containing silver trioxonitrate(V) solution was connected in series to another voltameter containing copper (II) tetraoxosulphate(VI) solution. When a current ri 0.200 ampere was passed through the solutions, 0.780g of silver was deposited. Calculate the
I. mass of copper that would be deposited in the copper voltameter
II. quantity of electricity used and the time of current flow. [Cu = 63.5 ; Ag = 108; 1F = 96500C]
(a)(i) Explain what is meant by acid anhydride and give one example
(ii) State three chemical properties of hydrochloric acid.
(b) Explain each of the following observations:
(i) Tetraoxosulphate (VI) acid can form two types of salts unlike trioxonitrate (V) acid.
(ii) Copper and iron react with concentrated H\(_2\)SO\(_4\) but only one of them reacts with the dilute acid.
(iii) On adding dilute H\(_2\)SO\(_4\) separately to zinc dust and zinc granules of the same mass, the dust produced more vigorous effervescence.
(c)(i) Define activation energy.
(ii) Sketch and label an energy profile diagram for the following reaction: A + B –> C + D; AH = xkJmol\(^{-1}\)
(iii) Explain why the heat of reaction of the mineral acids with sodium hydroxide is constant in value.
(d) Consider the reaction represented by the following equation:
Q\(_{(s)}\) \(\rightleftharpoons\) Q\(_{(l)}\) \(\Delta\) = xkJmol\(^{-1}\)
(i) State with reason which of Q\(_{(s)}\) and O\(_{(J)}\) has the higher entropy.
(ii) What will be the effect of decrease in temperature on the system at equilibrium?
(a) State three characteristic properties of
(i) electrovalent compounds;
(ii) alpha particles
(iii) catalysts
(b)(i) Write the electronic configuration of silicon (atomic number 14) and state the group to which it belongs in the Periodic Table.
(ii) State the type of chemical bonding between silicon and oxygen in SiO\(_2\)
(iii) A chip used in a microcomputer contains 5.72 x 10\(^{-3}\)g of silicon, calculate the number of silicon atoms in the chip.
[Si = 28; Avogadro constant = 6.02 x 10\(^{23}\) mol\(^{-1}\)]
(c) An element X belongs to the same group as sodium but is more reactive.
(i) Suggest with reason whether X would be a reducing or oxidizing agent.
(ii) What would be a suitable method of storing X in the laboratory?
(iii) Describe briefly what would be observed if a small piece of X were dropped into a trough of cold water which had been coloured with red litmus.
(iv) Write an equation to show how the oxide of X would react with dilute HCI.
(v) Suggest the likely colour of the salts of X
A visible change is observed when a strip of iron is placed in an aqueous solution of
- A. FeSO4
- B. ZnSO4
- C. CuSO4
- D. MgSO4
Which of the compounds will leave a metal residue when heated?
- A. Cu(NO 3 )2
- B. AgNO3
- C. K2CO3
- D. CaCO3
CuSO4 5H2O can be obtained from an aqueous solution of copper (ll) tetraoxosulphate (VI) by
- A. evaporation to dryness
- B. using chromatography
- C. precipitation
- D. crystallization
Equal amounts of marble chips are reacted separately with 100cm3 of hydrochloric acid of different concentrations . if all the marble chips reacted, which of the following remained the same in each case?
- A. Average rate of evolution of gas
- B. Total mass of gas evolved during the reaction
- C. Time taken for the reaction to reach completion
- D. lnitial reaction rates
What will happen if more heat is applied to the following system at equilibrium? X2(g) + 3Y 2(g) ⇌ 2XY3(g); ∆H =-kJmol -1
- A. the yield of XY 3 will decompose
- B. more of XY3 will decompose
- C. More of X2 will react
- D. the forward reaction will go to completion
Which of the following salt solutions will have a pH greater than 7?
- A. NaCl(aq)
- B. Na2CO3(aq)
- C. Na2SO4(aq)
- D. NaHSO4(aq)
Calculate the mass of sodium hydroxide in 5.00 dm3 of a 0.125 mol dm-3 solution [NaOH = 40 g mol]
- A. 0.0156 g
- B. 0.625 g
- C. 1.00 g
- D. 25. 0 g
Which of the following substances increases in mass when heated in air?
- A. Sodium chloride
- B. lodine crystals
- C. magnesium ribbon
- D. copper (ll) oxide
A substance is said to be hygroscopic if it absorbs?
- A. water from the atmosphere to form a solution
- B. heat from the surrounding
- C. carbon (IV) oxide from the atmosphere
- D. moisture from the atmosphere without dissolving
Which of the following acids forms normal salts only?
- A. Tetraoxosulphate (VI) acid
- B. Trioxosulphate (IV) acid
- C. Tetraoxophosphate (V) acid
- D. Trioxonitrate (V) acid
A major factor considered in selecting a suitable method for preparing a simple salt is its
- A. crystalline form
- B. melting point
- C. reactivity with dilute acids
- D. solubility in water
The gas given off when NH4Cl is heated with an alkali is
- A. H2
- B. CI2
- C. N 2
- D. NH 3


