(a)(i) Draw and label a simple cell for the electrolytic purification of copper.
(ii) Write can equation for the reaction at each electrode in (a)(i) above.
(iii) State with reason whether the Daniell cell is an electrolytic cell or an electrochemical cell.
(iv) What is the function of MnO\(_2\) in the Laclanche cell?
(b) Consider the following equation: MnO\(^-_4\) + 8H\(^+\) + xe\(^-\) \(\to\) Mn\(^{2+}\) + yH\(_2\)O. State the
(i) values of x and y;
(ii) oxidation state of Mn in MnO\(^-_4\).
(c)(i) List three factors that affect selective discharge of ions during electrolysis
(ii) State Faraday’s second law of electrolysis.
(iii) A voltameter containing silver trioxonitrate(V) solution was connected in series to another voltameter containing copper (II) tetraoxosulphate(VI) solution. When a current ri 0.200 ampere was passed through the solutions, 0.780g of silver was deposited. Calculate the
I. mass of copper that would be deposited in the copper voltameter
II. quantity of electricity used and the time of current flow. [Cu = 63.5 ; Ag = 108; 1F = 96500C]
Explanation
(a)(i)
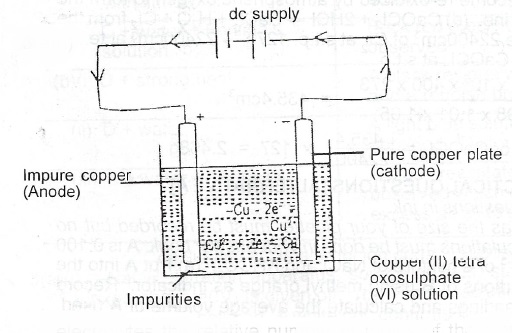
(ii) At the anode Cu\(_{(s)}\) - 2e\(^-\) -> Cu\(^{2+}\)\(_{(ag)}\); At the cathode Cu\(^{2+}\) + 2e \(\to\) Cu\(_{(s)}\)
(iii) Daniell cell is an electrochemical cell because chemical energy (from the reaction between the electrodes and the solution) is converted into electrical energy.
(iv) The function of MnO\(_2\) in the Leclanche cell Pure copper plate is to serve as depolarizer that is preventing the impure copper (cathode) hydrogen gas from adhering to the anode.
(b)(i) In MnO\(^-_4\) - + 8H\(^+\) + xe\(^-\) \(\to\) Mn\(^{2+}\) + yH\(_2\)O ; x = 5, y = 4
(ii) The oxidation of Mn in MnO\(^-_4\) is +7.
(c)(i) - Position of the ions in the electro-chemical series
-Concentration of the ions in the electrolyte
-Nature of the electrode.
(ii) Faraday's second law oxosulphate of electrolysis states that when the same quantity of electricity is passed through different electrolytes the relative number of moles of the elements discharged are inversely proportional to the charge on the ions of each of the elements respectively.
(iii) AgNO\(_3\) and CuSO\(_4\) ; Ag\(^+\) + e\(^-\) -> Ag\(_{(s)}\) ; Cu\(^{2+}\) + 2e\(^-\) -> Cu\(_{(s)}\)
96500C will liberate 108; It liberated 0.780
It = \(\frac{0.780}{108}\) x 96500C = 696.9C
(d)(i) 2 x 96500 C will liberate 63.50 ; 696.9C will liberate
= \(\frac{696.9}{2 \times 96500}\) x 63.50 = 0.2293g.
(ii) The quantity of electricity = 696.2 C.
but Q = It ; 0.200 x t = 696.9
t = \(\frac{696.9}{0.200}\) = 3484.5s; = 58.075 mins.

