(a) Suggest how the following liquid reagents can be suitably stored in the laboratory
(i) X which fumes in moist air;
(ii) Y which is slowly decomposed by sunlight in ordinary reagent bottles.
(b) State what is observed when aqueous ammonia is added to:
(i) litmus paper;
(ii) Pb(NO\(_3\))\(_2\) solution in drops until in excess
(iii) freshly precipitated AgCI in excess.
(c) A salt sample was suspected to be either Na\(_2\)CO\(_2\) or NaHCO\(_3\). A student who was required to identify it tested a portion for solubility in water and then for effect on litmus paper.
(i) What was observed in each case?
(ii) Give the reason why the student’s procedure was unsuitable
(iii) Describe briefly how you would have identified the salt.
Credit will be given for strict adherence to the instructions, for observations precisely recorded and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the i.itne time they are made.
C is a sample of copper (II) tetraoxosulphate (VI) crystals. Carry out the following exercises on C. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a) Put half of C in a test tube and heat strongly
(b) Make solution of about 10 cm\(^{-3}\) with the sécond half of C and divide it into three portions
(i) To the first portion, add Sodium hydroxide solution in drops and then in excess. Heat the mixture
(ii) To the second portion, add aqueous ammonia in drops and then in excess followed by few drops of moderately concentrated HCI.
(iii) To the third portion, add all the zinc dust provided and stir thoroughly until there is a visible change.
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but no account or expeimental procedure is required. All calculations must be done in your answer book.
A is 0.125 mol dm\(^3\) H\(_2\)SO\(_4\). B is a solution containing X g dm\(^{-3}\) of NaOH.
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is H\(_2\)SO\(_4\) +2NaOH\(_{(aq)}\) \(\to\) Na\(_2\)SO\(_4\) + 2H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided above, calculate the;
(i) amount of H\(_2\)SO\(_4\) in the average volume of A used
(ii) Concentration of B in mol dm\(^{-3}\)
(iii) value of X.
[H = 1: O = 16; Na = 23]
(c) Describe briefly a suitable laboratory procedure for obtaining pure water from the titration mixture. (No diagram is required)
a)(i) Give two uses of ammonia.
(ii) Name the process by which ammoniacal liquor can be obtained from coal and list two other products of the reaction
(iii) What type of reaction is involved in the conversion of ammoniacal liquor to (NH\(_4\))\(_2\)SO\(_4\) by dilute H\(_2\)SO\(_4\)?
(iv) Sketch and label an energy profile diagram to show the effect of presence of Pt/Rh on the reaction represented by the following equation: 4NH\(_3\) + 5O\(_2\) \(\to\) 6H\(_2\)O + 4NO; \(\Delta\)H = —907 kJmol\(^1\)
(b) Rock salt is an impure form of sodium chloride.
(i) Outline a suitable procedure for preparing a pure sample of sodium chloride from rock salt.
(ii) State two methods that can be used to prepare chlorine from rock salt. Write an appropriate equation in each case.
(c) Lead pigments were used in a water colour painting which turned black after prolonged exposure to an air pollutant. The original colour was restored by using H\(_2\)O\(_2\) which converted the black substance to a simple, white lead (II) salt.
(i) Which pollutant turned the painting black?
(ii) Write the formula of the black substance
(iii) What is the white salt?
(iv) State the role of H\(_2\)O in the restoration process.
(a)(i) State three methods of preparing salts, giving one example in each case of a salt so prepared.
(ii) What type of salt is each of the following? NaH\(_2\)PO\(_4\); (CH\(_3\)COO)\(_2\)Pb; KAI(SO\(_4\))\(_2\). 12H\(_2\)O.
(b)(i) Write an equation for the reaction between dilute HCI and a solution of AgNO\(_3\).
(ii) Explain why NaNO\(_3\) is preferred to AgNO\(_3\) in the preparation of oxygen by thermal decomposition of trioxonitrate (V) salts.
(iii) When silver wire was dipped into an aqueous solution of CuSO\(_4\), the wire remained intact but when the wire was replaced with zinc rod, the rod decreased in size. Give an explanation for this observation.
(c) When a sample of a crystalline salt X was exposed to air, there was a loss in mass.
(i) What phenomenon was exhibited by X?
(ii) Suggest two substances which X could be.
(iii) On heating 5.00 g of a fresh sample of X to constant mass, 1.80g was lost in the form of water vapour. Calculate the number of molecules of water of crystallization in one molecule of X. [H = 1.00; O = 16.00; Anhydrous form of X = 160 g mol\(^{-1}\)
(a) Explain in terms of the kinetic theory why a tyre should not be overinflated.
(b)The following results were obtained at room temperature in an experiment to verify one of the gas laws using a glass syringe:
|
Pressure (P) of air in syringe (atm) |
Volume (V) of air in syringe (cm\(^3\) | \(\frac{I}{V}\) |
| 0.100 | 10.00 | 0.100 |
|
0.125 |
8.00 | 0.125 |
|
0.150 |
6.60 | 0.150 |
|
0.175 |
5.60 | 0.179 |
|
0.200 |
4.80 | 0.208 |
| 0.225 | 4.40 | 0.227 |
(i) Plot a graph of P against \(\frac{1}{v}\), using 1 cm to represent 0.01 atm on the vertical axis and 1cm to represent 0.02 unit on the horizontal axis.
(ii) Which of the gas laws is in agreement with the results?
(c) The flow chart below represents the stages involved in the manufacture of H\(_2\)SO\(_4\).
+x +Conc. H\(_2\)SO\(_4\) +H\(_2\)O
S + O\(_2\) \(\to\) SO\(_2\) \(\to\) SO\(_3\) \(\to\) Y \(\to\) Conc H\(_2\)SO\(_4\)
stage I stage II stage III stage IV
(i) Name the process represented by the chart.
(ii) Identify reactant X and product Y.
(iii) What are the operating temperature and pressure at stage II?
(iv) Mention the stage which requires a catalyst and state the catalyst used.
(v) Give the reason why the SO\(_3\) produced in stage II is not dissolved directly in water to form the acid
(d) When K\(_4\)Cr\(_2\)C\(_7\) dissolves in water, the following equilibrium is established:
Cr\(_2\)O\(^{2-}_{7(aq)}\) + H\(_2\)O\(_{(l)}\) \(\to\) 2CrO\(^{2-}_{4(aq)}\) + 2H\(_{aq}\)
(i) State the colour observed on adding a few drops of dilute H\(_2\)SO\(_4\) to the system.
(ii) Explain your answer in (d)(1).
(iii) What principle is applicable to this explanation?
(a) What term is used to describe each of the following processes?
(i) Alkaline hydrolysis of fats and oils;
(ii) The conversion of glucose into ethanol by enzymatic action;
(iii) Thermal decomposition of higher petroleum fractions into lower molecular mass hydrocarbons in the presence of catalyst.
(b)(i) Write the structure and IUPAC name for one alkanoic acid with the molecular formula C\(_4\)H\(_8\)0\(_2\).
(ii) Arrange the following compounds in order of increasing boiling point: Butane; Butanoic acid; Methylpropane.
(iii) Give an explanation for your answer in (b)(ii).
(c)(i) Ethanol was used for preparing a gas X which decolorized bromine water. Identify X and describe briefly its laboratory preparation.
(ii) Write an equation to show how ethanol reacts with sodium
(iii) Give the reagent and reaction conditions for the conversion of ethanol into C\(_2\)H\(_5\)COOC\(_2\)H\(_5\).
(d) State the type of recction involved in each of the conversions indicated below:
(i)C\(_6\)H\(_6\)C\(_6\)H\(_5\)CH\(_3\)
(ii) nC\(_2\)H\(_4\) \(\to\) (CH\(_2\) – CH\(_2\)),
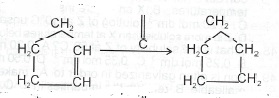
(iii) CH\(_3\)CH\(_2\)CH(OH)CH\(_3\) -> CH\(_3\)CH\(_2\)CCH\(_3\)
(iv) (C\(_6\)H\(_{10}\)O\(_5\)) -> C\(_6\)H\(_{12}\)O\(_6\).
(iv)
(a) Giving different examples, mention one metal in each case which produces hydrogen on reacting with
(i) dilute mineral acid
(ii) cold water;
(iii) steam;
(iv) hot, concentrated alkali.
(b) In an experiment, excess 0.50 mol dm\(^{-3}\) HCI was added to 1Og of granulated zinc in a beaker. Other conditions remaining constant, state how the reaction rate would be affected in each case, if the experiment was repeated using:
(i) 1.0 mol dm\(^{-3}\) HCI;
(ii) 8.0g of granulated zinc;
(iii) 10g of zinc dust;
(iv) a higher volume of 0.50 mol dm HCI;
(v) a reaction vessel dipped in crushed ice;
(vi) equal volumes of water and 0.50 mol dm\(^3\) HCI.
(c) Aluminium is extracted from its ore by electrolysis.
(i) Name the ore from which the metal is extracted.
(ii) State the role of molten cryolite in the extraction.
(iii) Describe in outline how the ore is purified before electrolysis
(iv) Calculate the current in amperes required to produce 18.0g of aluminium in 1.50 hours. [Al = 27.0; F = 96500C]
(d) Give the reason why
(i) aluminium, which is a reactive metal, is resistant to corrosion.
(ii) metals are generally good reducing agents.
(a) What is the shape of (i) p – orbital; (ii) a molecule of methane; (iii) a molecule of carbon (IV) oxide?
(b) Consider the following elements: Ne, S, CI, 0, Fe, Mg. State which of them
(i) exhibit(s) allotropy;
(ii) form(s) coloured ions;
(iii) is/are malleable;
(iv) consist(s) of molecules that are far apart at room temperature;
(v) form(s) hydrides by sharing electrons with hydrogen;
(vi) has/have complete outermost shell.
(c)(i) List three applications of radioactivity in different fields.
(ii) Explain clearly the difference between the following reactions involving electron loss from lead.
\(^{211} pb\) \(\to\) \(^{ 211}Bi\) + \(^0_{-1}\); Pb \(\to\) pb\(^{3+}\) _ 2e\(^-\)
(iii) Give one advantage and one disadvantage of nuclear power generation over the use of fossil fuels.
One of the methods used for combating air pollution is
- A. burning of solid waste
- B. provision of sanitary land fills
- C. legislation against some industrial practices
- D. scavenging of refuse dumps
Iron is often galvanized in order to
- A. make it more malleable
- B. remove the impurities in it
- C. protect it against corrosion
- D. render it passive
The energy value of petrol can be determined by a
- A. bomb calorimeter
- B. catalytic cracker
- C. fractionating column
- D. thermometer
The reaction of sucrose with dilute HCI produces
- A. an alkanoate
- B. glucose and fructose
- C. a black mass of carbon
- D. a polysaccharide
A suitable reagent for distinguishing between ethanoic acid ethanol is
- A. bromine water
- B. Fehling's solution
- C. sodium hydrogentrioxocarbonate (IV)
- D. ammoniacal silver trioxonitrate (V)
Which of the following types of alkanols undergo oxidation to produce alkanoic acids? 1. Primary alkanols 11. Secondary alkanols 111. Tertiary alkanols
- A. 1,11 and 111
- B. l and ll only
- C. ll only
- D. l only
Which of the following statements about soapless detergents is correct? They
- A. lather readily with hard water
- B. increase the surface tension of water
- C. cause hydrolysis of salts in water
- D. from a curdy precipitate with soft water
The reaction between ethene and chlorine to form 1, 2-dichloroethane is
- A. oxidation
- B. polymerization
- C. addition
- D. substitution
The following compounds are hydrocarbons except
- A. methylpropanoate
- B. 2-methylbutane
- C. benzene
- D. cyclohexane
When alkynes are hydrogenated completely they produce compounds with the general molecular formula
- A. CnHn
- B. CnH2n
- C. CnH2n+2
- D. CnH2n-2
Which of the following compounds can be represented by the molecular formula C2H6O?
- A. Propanal
- B. Ethanol
- C. Methanoic acid
- D. Glucose
Which of the following processes takes place during the production of margarine from vegetable oils?
- A. Esterification
- B. Hydrolysis
- C. Hydrogenation
- D. Saponification


