(a)Give two reasons why aluminium is preferred to copper for making overhead electric cables.
(ii) Describe briefly the electrolytic extraction of aluminium from purified bauxite.
(b) The diagram below represents an electrolytic cell used for the purification of copper.
(i) Which of the electrodes I and II increases in mass during the electrolysis? Give reasons for your answers
(ii) State with reason the site of oxidation
(iii) Identify III and explain why its colour does not change in intensity during the electrolysis.
(c) Calculate the current in amperes that will deposit 8.00 g of calcium from used CaCl\(_2\) in 1 hour 15 minutes. [Ca = 40.0; 1 Faraday = 96500C]
Explanation
(a)(i) Aluminium is preferred to copper for making overhead electric cables because aluminium: is lighter/has a lower relative density
-is more resistant to corrosion.
(ii) Extraction of aluminium from purified bauxite. A solution of the pure alumina in molten cryolite (Na\(_3\)AIF\(_6\)) is placed in the electrolytic cell made of iron lined with graphite which serves as the cathode. The anode consists of graphite rods dipping into the electrolyte (solution of alumina in molten cryolite). The cell is maintained at high temperature 900 ± 100°C. At the cathode: Al\(^{3+}\) + 3e\(^-\)m ---> Al ; At the anode: 2O\(^{2-}\) ---> O\(_2\) + 4e\(^-\). The molten aluminium collects at the bottom of the cell and is tapped off at intervals.
(b) Illustration of the cell used for the purification of copper.
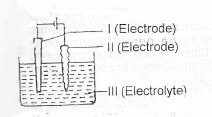
(i) Electrode I increases in mass during the electrolysis, because Cu\(^{2+}\) migrate to it. The Cu\(^{2+}\) is reduced to copper/Cu\(^{2+}_{(aq)}\)+ 2e --> Cu\(_{(s)}\) and the copper is deposited, leading to an increase in its mass.
(ii) Electrode II is the site of oxidation. Oxidation is electron loss and the reaction at this electrode is Cu -> Cu\(^{2+}\) + 2e\(^{2+}\)
(iii) Ill is CuSO\(_{4(aq)}\)/any soluble copper (II) salt. Its colour does not change in intensity because Cu\(^{2+}\) which is responsible for the colour is removed by the cathode (i) from the solution and is replaced by the anode.
(c) To calculate the current that will deposit 8.00g of calcium from fused CaCl\(_2\) in 1 hr. 15mins.
Ca\(^{2+}\) + 2e\(^{-}\) -> Ca.
40g of calcium will be deposited by (2 x 96500)C
8.00g of calcium will be deposited by \(\frac{8 \times 38600}{40}\) = 38600 C ; Let x represent 386000C
Q = It xc = 1 x 75 x 60 ;
Current (I) = \(\frac{38600}{75 \times 60}\) ; Current = 8.58A.

