State what you would see on;
(i) bubbling SO\(_2\) into acidified KMnO\(_4\) solution
(ii) mixing zinc dust with CuSO\(_4\) solution
(iii) adding concentrated HNO\(_3\) to freshly prepared FeSO\(_4\) solution.
(b) List two substances in case which, if added to dilute H\(_2\)SO\(_4\), would give you
(i) H\(_{2(g)}\)
(ii) ZnSO\(_{4(aq)}\)
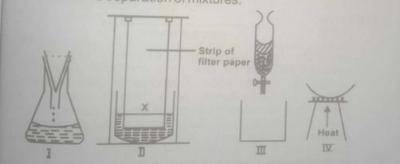
(c) The diagram labeled I to IV ABOVE illustrate different laboratory set-ups used in the separation of mixtures.
(i) Name the separation technique illustrated by each diagram
(ii) Which of the set-ups is used for concentrating dilute salt solutions, for the purpose of crystallization?
(iii) Which of the set-ups is used in obtaining clear water from muddy water?
(iv) Mention the set-up you would use to separate a polar solvent from a non-polar solvent
(v) State the modification you would make to the set-up labelled IV in order to use it for separating a mixture of NaCl and NH\(_4\)CI
Explanation
(i) On bubbling SO\(_2\) into acidified KMnO\(_4\) solution the solution becomes decolorized/ the solution disappears/discharged.
(ii) On mixing zinc dust with CuSO\(_{4(aq)}\) solution, the blue colour of the CuSO\(_{(aq)}\) gradually disappears/the solution becomes colourless. A reddish-brown or pink deposit is obtained.
(iii) On adding concentrated HNO\(_3\) to freshly prepared FeSO\(_4\) the solution, the solution changes colour from green to brown.
(b) Substances which, if added to dilute H\(_2\)SO\(_4\) would give;
(i) Hydrogen-magnesium/iron/zinc
(ii) ZnSO\(_{4(aq)}\) - ZnO/ZnCO\(_3\)/Zn
(c) (i) I - Filtration
II - Chromatography
III - Use of separating funnel/solvent extraction
IV - Evaporation
(ii) The set-up labelled IV is used for concentrating salt solutions.
(iii) The set-up labelled I is used in obtaining clear water from muddy water.
(iv) The set up labelled lIl is used for the separation of a polar solvent from a non-polar solvent.
(v) In order to use the set-up labelled (IV) for separating a mixture of NaCl and NH\(_4\)CI, cover the evaporating dish with an inverted funnel.

