All your burette readings (initial and final) as well as the size size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A is a solution containing 1.04 g HCl per 500 cm\(^3\) of solution. B was prepared by diluting 50.0 cm\(^3\) of a saturated solution of Na\(2\)CO\(_3\) at room temperature to 1000 cm\(^3\)
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^{3}\) portions of B using methyl orange as indicator. Repeat the titration to obtain consistent titres. Tabulate your results and calculate the average volume of acid used.
9b) From your results and information provided above, calculate the;
(i) concentration of A in moldm\(^{-3}\)
(ii) concentration of B in mol dm\(^{-3}\)
(iii) solubility of Na\(_2\)CO\(_3\) in mol dm\(^{-3}\)
(iv) volume of CO\(_2\) that would be liberated from 1 dm\(^3\) of B if the titration were carried out at s.t.p.
The equation for the reaction is Na\(_2\)CO\(_{3(aq)}\) + 2HCl\(_{(aq)}\) \(\to\) 2NaCl\(_{(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
[H = 1; C = 12; O = 16; Na = 23; Cl = 35.5; Molar volume of gas at s.t.p = 22.4 dm\(^3\)]
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. All tests, Observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is a mixture of two inorganic salts. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) Put all of C in a test tube and add about 10 cm\(^3\) of distilled water. Stir, filter, and keep the filtrate and the residue.
(b) Put the residue into a test tube and add about 5 cm\(^3\) of dilute HCl. Shake to dissolve
(i) To about 2 cm\(^3\) of the solution, add NaOH\(_{(aq)}\) in drops and then in excess
(ii) To another 2 cm\(^3\) portion of the solution, add NH\(_{3(aq)}\) in drops and then in excess.
(c) To about 2 cm\(^3\) portion of the filtrate, add few drops of dilute HNO\(_3\), and then AgNO\(_{3(aq)}\) a followed by aqueous NH\(_3\) in excess.
(a) State an indicator suitable for the titration of;
(i) dilute HCl and NaOH\(_{3(aq)}\)
(ii) dilute CH\(_3\)COOH and KOH\(_{(aq)}\)
(iii) dilute HCl and NH\(_{3(aq)}\).
Give a reason for your answer in each case.
(b) Calculate the volume of water that would be added to 50 cm\(^3\) of 0.10 mol dm\(^{-3}\) of HCI to dilute it to 0.010 mol dm \(^{-3}\)
(c) Name one gas that could be used to demonstrate the fountain experiment.
(i) Draw and label an energy profile diagram of an endothermic reaction. Indicate on your diagram the I. activation energy II. heat change, \(\Delta\)H.
(ii) Explain how the rate of reaction is affected by I. addition of a catalyst, II. increase in temperature.
(b)(i) Write an equation for the thermal decomposition of calcium trioxocarbonate (IV)
(ii) Determine the volume of carbon (IV) oxide measured at s.t.p. that would he produced by the thermal decomposition of 10g calcium trioxocarbonate (IV). [ Ca = 40; O = 16; C = 12 ]
(c)(i) Give one use cf each of the following forms of carbon: I. coal; II. wood charcoal; Ill. carbon (IV) oxide.
(ii) Write a balanced chemical equation to show what happens when each of the following compounds is heated strongly: I. NaNO\(_{3(s)}\) II. MgCO\(_{3(s)}\).
(d) Consider the following compounds: CaO, CaCO\(_3\), Ca(OH)\(_2\), NaOH Which of them is
(i) used in the manufacture of cement;
(ii) used to detect the presence of carbon (IV) oxide;
(iii) used to liberate carbon (IV) oxide when dilute acid is added;
(iv) hygroscopic;
(v) deliquescent?
i) What is the name of the process used for the industrial preparation of tetraoxosulphate (VI) acid?
(ii) State the catalyst used in (a)(i)
(iii) Show by means of bdanced chemical equations only, the industrial preparation of tetraoxosulphate (VI) acid from sulphur (IV) oxide.
(b)(i) Distinguish between dehydration and drying
(ii) Explain why concentrated tetraoxosulphate (VI) acid cannot be used to dry ammonia
(iii) What is the drying agent for ammonia?
(c)(i) Give one example of I. a chloride which is soluble in hot water, II. a trioxocarbonate (IV) which does not decompose on heating, Ill. an amphoteric oxide
(ii) List three methods for the preparation of salts
(iii) State one method for the recovery of salt from its solution.
(d)(i) State Gay Lussac’s law of combining volumes
(ii) Consider the reaction represented by the following equation: C\(_2\)H\(_{4(g)}\) + 3O\(_{2(g)}\) \(\to\) 2H\(_2\)O\(_{(g)}\) + 2CO\(_{2(g)}\). What is the volume of oxygen required for the complete combustion of 12.5 cm\(^{3}\) of ethene?
(a) Write an equation in each case to represent the (i) \(\beta\)-decay of \(^{24}_{11}Na\) to give Mg.
ii) reaction of sodium with cold water.
b)(i) State two differences between reaction (a)(i) and (ii)
ii) State two applications of the type of reaction represented in(a)(i)
(c) Consider the reaction represented by the equation:
Mg\(_{(s)}\) + 2HCI\(_{(aq)}\) \(\to\) MgCl\(_{(aq)}\) + H\(_{(q)}\)
(i) Name the type of reaction involved
(ii) Give two ways by which the reaction could be made faster.
(iii) What volume of hydrogen gas would be produced from 6.0 g of the magnesium? [ H = 1; 1 mole of gas occupy 22.4 dm\(^3\) at s.t.p. ]
(d) What is (i) an electrolyte?; (ii) electrolysis?
(e)(i) Give one metal that is extracted using electrolytic process.
(ii) Name the ore of the metal.
(iii) What is the substance discharged at each electrode when dilute NaCI is electrolysed using graphite electrodes?
(iv) Why would aqueous NaCI conduct electricity but solid NaCI would not?
(v) Give one industrial use of NaCI.
(a)(i) What is 2 homologous series?
(ii) Give two homologous series present in petroleum
(iii) Give one example of a compound belonging to each of the homologous series in (a)(ii).
(iv) Name two fractions obtained from the fractional distillation of petroleum
(v) Why is there a gradual change in the physical properties of petroleum fractions?
b) Write a two-step balanced chemical equation for the reaction of (i) ethanol with excess concentration traoxosulphate (VI) acid at high temperature
(ii) excess ethanol with concentrated tetraoxosulphate (VI) acid at lower temperature.
(c) An organic compound of relative molecular mass 46, on analysis vas ound to contain 52.0% carbon, 13.3% hydrogen and 34.7% oxygen.
(i) Determine its I. empirical formula, II. molecular formula.
(ii) Draw two possible structures of the compound and name one of them [O = 16; C = 12; H = 1 ]
a) Define the following in term:. of electron transfer: (i) oxidation; (ii) reduction.
(b)(i) Determina the oxidation stale of phosphorus in each of the following structures: I. POCI\(_3\) II. PH\(_3\).
(ii) State with reasons whether the following compounds will form acidic, neutral or basic aqueous solutions: I. NaNO\(_3\) II. Na\(_2\)H\(_4\)CI; Ill. Na\(_2\)CO\(_3\).
(c) Consider the set-up
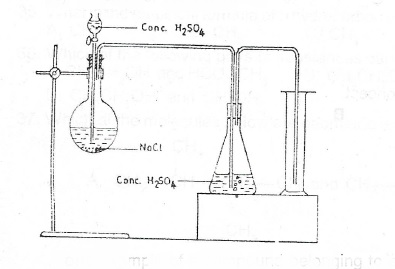
(i) What is the gas produced in the experiment illustrated by the set-up above?
(ii) Name the method of collection of gas
(iii) Give a reason for your answer in (c)(ii) above
(iv) State the function of the concentrated H\(_2\)SO\(_4\) in the conical flask
(v) Give of collection of the gas one I. physical property; II. chemical property of the gas
(vi) State one chemical test to identify the gas.
(d) A 4.3 g hydrated sodium tetraoxosulphate (VI) (Na\(_2\)SO\(_4\).xH\(_2\)O) was heated to remove the water of crystallization. The remaining anhydrous salt had a mass of 2.12 g. Calculate the value of x in the t I hydrated salt. [H = 1; O = 16; Na = 23; S = 32 ]
(a) Define the following terms:(i) Saturated solution; (ii) Solubility.
(b) In an experiment to determine the solubility of a given salt Y, the following data were provided:
Mass of dry empty dish = 7.16 g
Mass of dish + saturated solution of salt Y = 17.85 g
Mass of dish + salt Y = 9.30 g Temperature of solution = °C
Molar mass of salt Y = 100
Density of solution Y = 1.00 g cm\(^{-3}\) Calculate the solubility of salt Y in
(i) g dm\(^{-3}\) of solution, (ii) mol dm\(^{-3}\) of solution.
(c) State the type of bond broken on melting each of the following substances: (i) NaCI\(_{(s)}\) (ii) CO\(_{2(s)}\) (iii) SiO\(_{2(s)}\) (iv) Al\(_{(s)}\)
(d) Explain the following observations: (i) the chemical reactivity of alkali metals increases down the group; (ii) Mg has higher melting point than Na; (iii) K is a better reducing agent than Na.
(e)(i) What are isotopes? (ii) Lithium exists as \(^6_3\)Li and \(^7_3\)Li in the ratio 2:25. Calculate the relative atomic mass of the lithium.
Which of the these metals will not liberate hydrogen from dilute HCI?
- A. Copper
- B. Iron
- C. Magnesium
- D. Zinc
What volume will 0.5 g of H2 occupy at s.t.p? [H = 1; 1 mole of a gas occupy 22.4 dm3 at s.t.p.]
- A. 2.24 dm3
- B. 5.60dm3
- C. 11.20dm3
- D. 44.80dm3
Pig-iron is brittle because it contains
- A. a high percentage of carbon as impurity
- B. calcium trioxosilicate (IV)
- C. unreacted haematite
- D. undecomposed limestone
The following substances are heavy chemicals except
- A. sodium trioxocarbonate (IV)
- B. tetraoxosulphate (VI) acid
- C. lead (lV) tetraethyl
- D. sodium hydroxide
Which of the following methods can be used to separate a mixture of two miscible liquids with different boiling points?
- A. Decantation
- B. Distillation
- C. Evaporation
- D. Filtration
from the following ores is iron extracted? I. Haematite ll. Bauxite III. Magnetite IV. Cassiterite
- A. l and ll only
- B. l and lll only
- C. l, ll and lll only
- D. l, ll, lll and lV
Glucose reduces Fehling’s solution on warming to
- A. copper (l) oxide
- B. copper (ll) oxide
- C. copper (l) chloride
- D. copper (ll) hydroxide
Which of the following compounds reacts readily with sodium to liberate hydrogen?
- A. CH3CH2CH3
- B. CH3COOH
- C. CH3 CH2CHO
- D. CH3 CH(OH)CH3
Consider the reaction below: Vegetable oil \(\frac{H2;NI catalyst}{High temperature}\) product. The reaction is applied in the manufacture of?
- A. drugs
- B. margarine
- C. paraffin wax
- D. soapy detergents
Hydrocarbons which react with ammoniacal copper (l) chloride solution conform to the general molecular formula
- A. CnH2n
- B. CnH2n
- C. CnH2n+2
- D. CnH2n-2
Alkenes undergo the following reactions except
- A. addition
- B. hydration
- C. polymerization
- D. substitution
Which of the following pairs of substances can be distinguished by use of NaHCO3 solution?
- A. CH 3CH2OH and HCOOCH3
- B. CH 3CH2OH and CH3COOH
- C. CH3COOH and HCOOH
- D. CH 3CH2OH and CH3OH


