(a)(i) What is 2 homologous series?
(ii) Give two homologous series present in petroleum
(iii) Give one example of a compound belonging to each of the homologous series in (a)(ii).
(iv) Name two fractions obtained from the fractional distillation of petroleum
(v) Why is there a gradual change in the physical properties of petroleum fractions?
b) Write a two-step balanced chemical equation for the reaction of (i) ethanol with excess concentration traoxosulphate (VI) acid at high temperature
(ii) excess ethanol with concentrated tetraoxosulphate (VI) acid at lower temperature.
(c) An organic compound of relative molecular mass 46, on analysis vas ound to contain 52.0% carbon, 13.3% hydrogen and 34.7% oxygen.
(i) Determine its I. empirical formula, II. molecular formula.
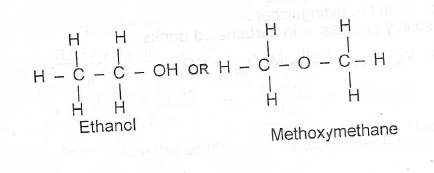
(ii) Draw two possible structures of the compound and name one of them [O = 16; C = 12; H = 1 ]
Explanation
(a)(i) Homologous series is a series are family of organic compounds with a general molecular formula, similarity in property and differing from successive member by CH\(_2\) of 14 a.m.u.
(ii) Alkanes, alkenes, cycloalkar les and aromatics.
(iii) Alkane — methane; ethane, etc.
Alkene — ethene, propene, butene, etc
Cycloalkanes — cyclohexane,
cyclopropane Aromatic — benzene.
(iv) Gas Petroleum ether Gasoline (petrol) Gas oil/diesel oil Lubricating oil Biturhen Kerosine.
(v) Because of a steady change (increase or decrease) in molecular mass of tho constituents.
(b)(i)
I. C\(_2\)H\(_5\)OH\(_{(l)}\) + H\(_2\)SO\(_4\)\(_{(l)}\) \(\to\) C\(_2\)H\(_5\)HSO\(_4\)\(_{(l)}\) + H\(_2\)O\(_{(l)}\)
II. C\(_2\)H\(_5\)HSO\(_4\)\(_{(l)}\) \(\to\) C\(_2\)H\(_4\)\(_{(g)}\) + H\(_2\)SO\(_4\)\(_{(l)}\)
(ii) I. C\(_2\)H\(_5\)OH\(_{(l)}\) + H\(_2\)SO\(_4\)\(_{(l)}\) ---> C\(_2\)H\(_5\)HSO\(_4\)\(_{(l)}\) + H\(_2\)O\(_{(l)}\)
II. C\(_2\)H\(_5\)HSO\(_4\)\(_{(l)}\) \(\to\) C\(_2\)H\(_5\)OC\(_2\)H\(_5\)\(_{(l)}\) + H\(_2\)SO\(_4\)
(c)(i)
(I) C H O
52.0 13.3 34.7
12 1 16
4.33 13.3 2.17
2.17 2.17 2.17
1 6 1
Emperica formula = C\(_2\)H\(_6\)O
(II) (C\(_2\)H\(_6\)O)\(_n\) = 46
(12 x 2 + 6 x 1 + 16)\(_n\) = 46
46\(_n\) = 46
n = \(\frac{46}{46}\) = 1
molecular formula = C\(_2\)H\(_6\)O
(ii)