Burette readings(initial and final) must be given to two decimal places. Volume of pipette user must also be recorded but on account of experimental procedure is required. All calculations must be done in your answer book. A is O.050 mol dm\(^{-3}\) of acid HX. Bis a solution of NaOH containing 0.025 moles per 250 solutions.
(a) Put A into the burette and titrate it against 20.00 cm\(^3\) or 25.00 cm\(^3\) portions B using phenolphthalein as indicator. Tabulate your readings and calculate the average volume or A used.
(b) your results and the information provided above, calculate the;
(i) amount of acid in the average
(ii) amount of base in 20.00 cm\(^3\) or 25.00 cm\(^3\);
(iii) mole ratio of acid to base
(c) Write a balanced chemical equation for the reaction between the acid H\(_y\)X and the base NaOH
(d) State the basicity of the acid H\(_y\)X.
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly centered in your answer book, at the time they are made.
C is a mixture of two salts. Carry outline following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) Put C into a beaker and add about 10 cm\(^3\) of distilled water, stir the mixture, and filter. Test the filtrate with litmus paper. Keep the residue and the filtrate.
(b)(i) To about 2 cm\(^3\) of the filtrate, add few drops of aqueous HNO\(_3\) followed by AgNO\(_{3(aq)}\)
(ii) Add excess NH\(_3\) solution to the resulting mixture.
(c) To about half of the residue from (a) above, add about 5cm\(^3\) of dilute HNO\(_3\) in drops. Divide the resulting solution into two equal portions.
(d)(i) To the first portion add ammonia solution in drops and then in excess.
(ii) To the second portion add dilute hydrochloric acid.
(a) In the laboratory preparation of crystals of CuSO\(_4\), a green powder Q was added to dilute H\(_2\)SO\(_4\), and stirred. Effervescence occurred and a gas R was given off which turned lime water milky. Excess Q was removed from the mixture. The solution of Cu\(_2\)SO\(_4\) was concentrated to half its original volume and allowed to stand.
(i) What is substance Q?
(ii) Name gas R
(ii) Why was excess Q used?
(iv) How would you know that the reaction is complete?
(v) What method was used to remove excess Q? (vi) Why was the solution of CusO\(_4\) not heated to dryness?
(b) Name the reagent(s) used for testing each of the following substances in the laboratory: (i) Water; (i) Primary alkanol.
(a)(i) Define hard water
(ii) Name two substances responsible for hardness in water
(iii) Give two methods for the removal of hardness in water.
(b)(i) What are the raw materials require for the manufacture of tetraoxosulphate (VI) acid by the contact process?
(ii) Write an equation for tr reaction that requires a catalyst in the contact process (iii) State the catalyst used in (b)(ii).
(c)(i) Give two uses of sodium tetraoxosulphate (VI) Consider the reaction represented by the following equation:
Na\(_2\)SO\(_4\).10H\(_2\)O\(_{(s)}\) \(\to\) Na\(_2\)SO\(_4\).H\(_2\)O + 9H\(_2\)O\(_{(g)}\)
(ii) What name is given to this type of reaction?
(iii) Calculate the solubility of Na\(_2\)CO\(_3\) at 25°C. if 30.0 cm\(^3\) of its saturated solution at that temperature gave 1.80 g of the anhydrous salt. [C = 12, 0 = 16, Na = 23]
(d)(i) Define the term activated complex
(ii) State one reason why a collision may not produce a chemical reaction
(iii) The formation of water gas is represented by the following equation:
C\(_{(s)}\) + H\(_2\)O\(_{(g)}\) \(\to\) CO\(_{(g)}\) + H\(_{2(0)}\) \(\Delta\)H= + 131 kJmol\(^{-1}\)
Draw an energy profile diagram for the reaction showing the I. activated complex, II. enthalpy of reactants.
(a)(i) What are acidic oxides?
(ii) Give one example of each of the following oxides: I. acidic oxide; II. basic oxide; III. amphoteric oxide; IV. neutral oxide
(b)(i) Define each of the following terms; I. Heat II. Heat of neutralization
(ii) Weite the above an equation to illustrate each of the terms in (b)(i) above
(ii) Given that the standard heat of combustion of butane (C\(_4\)H\(_{(10)}\) is + 5877 kJmol\(^{-1}\), calculate the heat of 14.5 g butane. [ H = 1, = 12 ]
(c) (i) Name two allotropes of sulphur
(ii) State one difference between the two allotropes.
(d)(i) Give two characteristics of noble eases
(ii) State one use each of I. He; II. Ar.
(e) State what is observed on warming ammonium trioxonitrate (V) with sodium hydroxide.
(a)(1) Define covalent bond.
(ii) Give two properties of covalent compounds
(ii) With the aid of a diagram, show how ammonia molecule is formed
(iv) Illustrate with a diagram the formation of ammonium ion?
(v) What type of bond(s) exist(s) in I. ammonia, H. ammonium ion? (\(_1\)H\(_7\)N)
(b)(i) Write three subatomic particles with their corresponding relative masses. CH\(_2\)OH
(ii) Name the possible states in which water can exist.
(c) (i) State Graham’s law of diffusion
(ii) Arrange the following gases, He, CH\(_4\) and N\(_2\) in order of increasing rates of diffusion. Give a reason for the order. [ H = 1, He = 4, C = 12, N = 14 ]
(d) Draw the structures of the following compounds:
(i) 2,3-dimethylbutane;
(ii) 1,4-dibromocyclohexane.
(a) Consider the following reaction sequence.
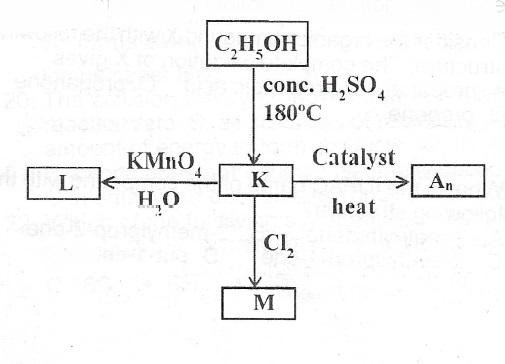
(i) What process leads/tathe formation of K?
(ii) Write the formula of K.
(iii) Write the structural formula of L and name L.
(iv) Name A\(_n\)
(v) Write the structure of M and name M.
(b)(i) What are carbohydrates?
(ii) Give one example each of a I. monosaccharide; II. disaccharide; III. polysaccharide.
(c) Consider the following structure of a simple sugar :
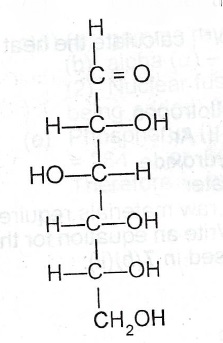
(i) Which functional group makes the compound a reducing agent?
(ii) State what would C = O be observed when I. the compound is mixed with Fehling’s solution and boiled;
I. few drops of concentrated H\(_2\)SO\(_4\) is added to the sample of the compound. H—C—OH
(iii) Write an equation for the reaction in (c)(ii)(II).
(d) A hydrocarbon Z with molecular mass 78 on combustion gave 3.385 g of CO\(_2\) and and 0.692 g of H\(_2\)O. Determine the molecular formula of Z. [ H = 1, C = 12, O = 16]
(a)(i) Define in terms of electron transfer I. oxidizing agent; II. reducing agent.
(ii) Write a balanced equation to show that carbon in a reducing agent.
(iii) State the change in oxidation number of the specie that reacted with carbon in (a)((ii).
(b) A gas X has a vapour density of 32. It reacts with sodium hydroxide solution to form salt and water only. It decolourizes acidified potassium tetraoxomanganate (VII) solution and reacts with H\(_2\)S to form sulphur. Using the information provided:
(i) identify gas X; (ii) state two properties exhibited by X;
(iii) give two uses of X.
(c) Consider the following substances: sodium; lead (II) iodide; hydrogen; magnesium; oxygen. Which of the substances
(i) conducts electricity?
(ii) is produced at the cathode during electrolysis of H\(_2\)SO\(_{4(aq)}\)?
(iii) corresponds to the molecular formula A\(_2\)?
(iv) is an alkaline earth metal?
d)(i) Define the term salt.
(ii) Mention two types of salt
(iii) Give an example of each of the salts mentioned in (d)(ii) above.
(e) In a neutralization reaction, dilute tetraoxosulphate (VI) acid completely reacted with sodium hydroxide solution.
(i) Write a balanced equation for the reaction
(ii) How many moles of sodium hydroxide would be required for the complete neutralization of 0.50 moles of tetraoxosulphate (VI) acid?
(a) State (i) Pauli’s Excusion principle;
(ii) Hund’s rule of maximum multiplicity.
(b)(i) Write the electronic configuration of each of the following ions of copper: I. Cu\(_+\) II. Cu\(_{(2+)}\) [\(_{29}Cu\)]
(ii) Give the number of unpaired-electrons in each of the ions in (b)(i) above.
(iii) State the type of reaction represented by the following equation:
2Cu\(^+_{(aq)}\) \(\to\) Cu\(^{2+}_{(aq)}\) + Cu\(_{(s)}\)
(iv) Write the formula of one compound of Cu+.
(c)(i) Name the type of radiation that will I. penetrate lead block; II. be stopped by thin paper
(ii) Give the charge on each of the radiations mentioned in I(c)(i) above.
(iii) What term is used to describe each of the following nuclear processes?
I. Combination of two lighter nuclei to form a heavy nucleus II. Splitting of a heavy nucleus into two or more lighter nuclei
III. Time required for one-half of the atoms of a radioactive substance to decay.
(d) Arrange the following ions in order of increasing size. Give a reason for your answer in each case. I. Li\(^{+}\), K\(^{+}\), Na\(^{+}\); II. O\(^{2-}\), F\(^{-}\), N\(^{3-}\)
(e) Determine the percentage composition of phosphorus and oxygen in phosphorus (V) oxide [ P = 31, O = 16 ]
Which of the following alloys is mainly used in making statues?
- A. Brass
- B. Bronze
- C. Duralumin
- D. Steel
Aluminium is a good roofing material because it
- A. is strong and does not conduct heat and electricity
- B. readily absorbs light
- C. forms an oxide film over its surface which prevents corrosion
- D. is flexible and can conduct heat
Chemicals that are produced in small quantities and with very high degree of purity are
- A. bulk chemicals
- B. fine chemicals
- C. heavy chemicals
- D. light chemicals
Which of the following substances causes the depletion of the ozone layer in the atmosphere?
- A. Carbon (IV) oxide
- B. Chlorofluorocarbon
- C. Sulphur (IV) oxide
- D. Hydrocarbons
A solution of a salt was acidified with HCI when a few drops of BaCI2 solution were added, a white precipitate was formed. Which of the following anions is present in the salt?
- A. CO2- 3
- B. NO- 3
- C. SO2- 4
- D. SO2- 3
A hydrocarbon containing 88.9% carbon has the empirical formula [H = 1 C = 12]
- A. CH
- B. CH2
- C. C2H3
- D. C2H5
Arrange the following compounds in order of increasing boiling point
l.CH 3CH2CH2OH
II.CH 3CH 2OH
III.CH3CH2CH2CH2OH
IV. CH3CH 2CH2CH 2CH 3
- A. I < II < IV < III
- B. IV < III < II < I
- C. I < II < III < IV
- D. IV < I < II< III
What is the main type of reaction alkenes undergo?
- A. Addition
- B. Condensation
- C. Elimination
- D. Substitutio
The hydrolysis of groundnut oil by potassium hydroxide is known as
- A. hydrogenation
- B. saponification
- C. esteriffication
- D. neutralization
When ethanol is heated with excess concentrated tetraoxosulphate (VI) acid, the organic product formed is
- A. ethanal
- B. ethanoic acid
- C. ethane
- D. ethene
Which of the following compounds would not give a precipitate with ammoniacal AgNO3 solution?
- A. CH3 C≡CCH3
- B. HC≡CH
- C. CH3C≡CH
- D. CH3CH2C≡CH
A mixture of kerosene and diesel oil can be separated by
- A. crystallization
- B. distillation
- C. precipitation
- D. sublimation


