The electron configuration of \(_{26}Fe^{3+}\) is?
The correct answer is: D
Explanation
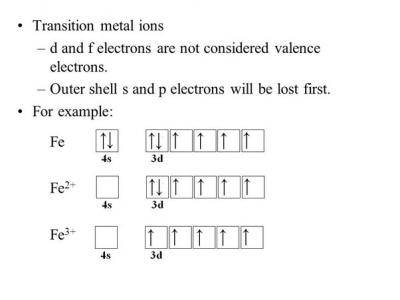
The neutral Iron atom has the electric configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d6.to write the electronic config. of Fe3+ we have to subtract 3 electrons from the outermost shell which is 4s and 3d orbitals. Thus we obtain the electronic configuration for Fe3+ as:
1s2 2s2 2p6 3s2 3p6 4s0 3d5
OR
[AR] 4s0 3d5.


