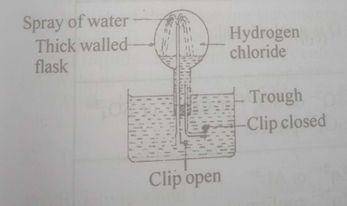
(a) Consider the following diagram ABOVE.
(i) Give the name of the experiment.
(ii) What does the experiment demonstrate?
(iii) Name one gas that could be used in place of HCl gas.
(iv) What colour could be observed in the flask during the spray of water?
(v) Could the gases used in the experiment be collected over water?
(vi) Explain briefly your answer in (a)(v).
(b) A solid substance U when strongly heated decomposes to give a white solid V and carbon (IV) oxide. When water is added to V, W is produced W can be used to test for carbon (IV) Oxide. Identify U, V and W.
Explanation
(a)(i) Fountain experiment
(ii) To demonstrate high solubility of hydrogen chloride gas
(iii) Ammonia
(iv) No colour/colourless
(v) No
(vi) The gases are highly soluble in water
(b) U - CaO\(_3\)/Calcium trioxocarbonate (IV)
V - CaO/Calcium oxide
W - Ca(OH)\(_3\)/Calcium hydroxide/Lime water

