All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
A is 0.200 moldm\(^3\) of HCl. C is a solution containing 14.3g of Na2CO\(_{3}\).xH\(_2\)O in 500 cm\(^3\) of solution.
a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0cm\(^3\) portions of C using methyl orange as indicator. Repeat the titration to obtain Consistent titre values. Tabulate vour results and calculate the average volume of A used. The equation for the reaction is;
Na\(_2\)CO\(_3\) . xH\(_2\)O + 2HCl\(_{(aq)}\) \(\to\) 2NaCl\(_{(aq)}\) + CO\(_{2(g)}\) + (x + 1)\(_3\)H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided. calculate the:
(i) concentration of C in moldm\(^{-3}\)
(ii) concentration of C in gdm\(^{-3}\)
(iii) molar mass of Na\(_2\)CO\(_3\) . xH\(_2\)O
(iv) the value of x in Na\(_2\)CO\(_3\) . xH\(_2\)O. [H =1.0; C = 12.0; O = 16.0; Na = 23.0]
Credit will be given for strict adherence to the instruction, for observations precisely recorded and for accurale references. All tests. obsenations and influences must be cleary entered in the booklet in ink at the same time they are made.
All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
F is 2 mixture of two inorganic salts. Carry out the following exercises on F. Record your observations and identify any gas(es) evolved. State the conclusions you draw from the result of each test.
(a) Put all of F in a beaker and add about 10 cm\(^3\) of distilled water. Stir well and filter. Keep the filtrate and the residue.
(b)(i) To about 2cm\(^3\) of the filtrate. add NaOH\(_{(aq)}\) in drops and then in excess.
(ii) To another 2cm\(^3\) portion of the solution, add a few drops of NH3\(_{(aq)}\) in drops and then in excess.
(c) To about 2cm\(^3\) of the solution, add a few drops of HNO\(_{3(aq)}\) followed by few drops of the drops of AgNO\(_{3(aq)}\)
(d)(i) Put all the residue into a clean test-tube and add HNO\(_{3(aq)}\)
(ii) To a portion of the solution from (d)(i)) add NaOH\(_{(aq)}\) in drops and then in excess.
All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
State what would be observed if the following reactions are carried out in the laboratory:
(i) methyl orange is dropped into a solution of lime juice:
(ii) hydrogen sulphide gas is bubbled through Iron (III) chloride solution:
(iii) sulphur (IV) oxide gas is bubbled into acidified solution of KMnO\(_4\):
(iv) ethanoic acid is added to a solution of Ka\(_2\)CO\(_3\)
(a)(i) Describe briefly how trioxonitrate (V) ions could be tested for in the laboratory.
(ii) State two uses of each of the following compounds: I. sodium chloride; II. sodium trioxocarbonate (IV).
(b) Write balanced equations for the reactions involved in the extraction of iron in the blast furnace.
(ii) State Faraday’s first law of electrolysis.
(iii) State two applications of electrolysis.
(c) Concentrated tetraoxosulphate (VI) acid is added to sugar crystals in a beaker. State what would be observed. Explain briefly your answer.
(d) Write an equation for the reaction of zinc powder with:
(i) dilute tetraoxosulphate (VI) acid;
(ii) concentrated tetraoxosulphate (VI) acid.
(e) What property of concentrated tetraoxosulphate (VI) acid is shown in (d)(ii)
(a)(i) Describe briefly the industrial preparation of ammonia.
(ii) Write a balanced equation for the reaction in (a)(i).
(iii) State one way of increasing the yield of ammonia in 4(a)(i).
(iv) State two uses of ammonia.
(b) Describe briefly, one chemical test for each of the following gases in the laboratory: (i) hydrogen; (ii) carbon (IV) oxide; (iii) oxygen.
(c)(i) State the composition of water gas.
(ii) List two uses of water gas.
(d) Describe briefly a simple experiment to determine the type of hardness in a sample of water.
(a) Write the molecular formula of X.
(i) What type of reaction is represented by the equation?
(ii) Consider the following reaction equation: C\(_{12}H_2\) \(\to\) X + C\(_8\)H\(_{18}\)
(iii) Draw the structure of two isomers of X.
(iv) Name the isomers drawn in (a)(iii).
(v) Write a balanced equation for the reaction between X and hydrogen.
(b) Describe one test for fats.
(c) Sulphur (IV) oxide is converted to tetraoxosulphate (VI) acid according to the following equation: 2SO\(_{2(g)}\) + O\(_{2(g)}\) + 2H\(_2\)O\(_{(l)}\) \(\to\) 2H\(_2\)OSO\(_{4(aq)}\). If 1.5 moles of oxgen reacts with sulphur (IV) oxide, calculate the mass of tetraoxosulphate (VI) acid produced. [H = 1.0; O = 16.0; S = 32.0].
(d) Consider the following neutralization reaction:
CH\(_3\)COOH + NaOH \(\to\) CH\(_3\)COONa + H\(_2\)O; \(\bigtriangleup\)H\(_1\)
CH\(_3\)COOH + NH\(_4\)OH \(\to\) CH\(_3\)COONH\(_4\) + H\(_2\)O; \(\bigtriangleup\)H\(_2\)
NaOH + HCl \(\to\) NaCl + H\(_2\)O \(\bigtriangleup\)H\(_3\)
(i) Arrange the enthalphy changes for the reactions in order of increasing magnitude.
(ii) Explain briefly your order in (d)(i).
(e) Consider the following substances. Cu\(_{(s)}\), BeCl\(_2\), NaH\(_{(s)}\), HF\(_{(s)}\)and CCl\(_{4(l)}\). State the substance(s) which;
(i) can conduct electricity;
(ii) is/are soluble in water.
(a)(i) 1. State the periodic law.
2. What is meant by the term periodic property of elements?
(ii) List three properties of an element which show periodicity.
(iii) Explain briefly how each of the properties listed in (a)(i) in varies across the period.
(b) Defulle relative atomic mass.
(c)(i) What phenomenon is exhibited by an element Z which exist as \(^{35}_{17}Z\) and \(^{37}_{17}X\)
(ii) What accounts for the difference in the mass numbers of the element Z?
(iii) Calculate the relative atomic mass of Z if the percentage abundance of \(^{37}_{17}Z\) is 75%
(d)(i) State the method used for collecting each of the following gases: I. CO II. HCI III. H\(_2\)
(ii) Give a reason for your answer stated in (d)(i) I and II
(a)(i) What is an acid-base indicator?
(ii) Give one example of an acid-base indicator.
(b) State the property exhibited by nitrogen(IV) oxide in each of the following equations:,
(i) 4Cu + 2NO\(_2\) -> 4CuO + N\(_2\) (ii) H\(_2\)O + 2NO\(_2\) –> HNO\(_3\) + HNO\(_2\)
(c)(i) Define enthalpy of combustion..
(ii) State why the enthalpy of combustion is always negative.
(d)(i) Distinguish between a primary cell and a secondary cell.
(ii) Give an example of each of the cells stated in I (d)(i).
(e) Define the term mole.
(f) Calculate the amount of hydrochloric acid in 40.0 cm\(^3\) of 0.40 moldm\(^{-3}\) dilute HCl.
(g) Name two substances which can be used as electrodes during the electroylsis of acidified water.
(h) List two forces of attraction that can exist between covalent molecules.
(i) Name the products formed when butane undergoes incomplete combustion.
(j) Write the electron configuration of \(_{26}\)Fe\(^{3+}\)
Petroleum is a non-renewable source of energy because it
- A. is formed naturally
- B. is cheap
- C. can be recycled after use
- D. cannot be regenerated once used up
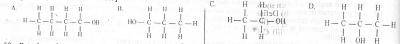
A primary alkanol has a molecular mass of 60. What is the structural formula of the compound? [C= 12.0, H= 1.0, O= 16.0)
- A. A
- B. B
- C. C
- D. D
The real harmful effect of the release of chlorofluorocarbons into the atmosphere is that it eventually causes
- A. excessive ultraviolet light from the sun to reach the earth surface
- B. excessive release of infrared light from the sun to the earth surface
- C. corrosive effect of the chemicals on humans and animals
- D. acidic effect of chemicals on humans
The IUPAC name for the compound \(ClCH_{2}CH_{2}OH\) is
- A. 1- chloropropanol
- B. 2-chloropropanol
- C. 1-chloroethanol
- D. 2-chloroethanol
The condensation of two units of glucose will produce a dissacharide with the formula \(C_{12}H_{22}O_{11}\) which is
- A. sucrose
- B. maltose
- C. galactose
- D. mannose
Fats and oils can be obtained from any of the following sources except
- A. paraffin oil
- B. animals
- C. groundnuts
- D. palm kernel
Which of the following pairs of gases are pollutants from car exhaust?
- A. Nitrogen(II) oxide and Carbon(IV) oxide
- B. Carbon(IV) oxide and Nitrogen
- C. Carbon(II) oxide and Sulphur(IV) oxide
- D. Carbon(II) oxide and oxygen
The name of the compound \(C_{4}H_{9}COOC_{3}H_{7}\) is
- A. propyl pentanoate
- B. pentyl propanoate
- C. butyl propanoate
- D. propyl butanoate
Classification of alkanols is based on the
- A. number of carbon atoms present in the compound
- B. molecular mass of the compound
- C. molecular formula of the compound
- D. number of alkyl groups bonded to the carbon having the hydroxyl group
Calculate the mass of copper deposited if a current of 0.45A flows through \(CuSO_{4}\) solution for 1hour 15mins. [Cu=64.0, S= 32.0, O =16.0, 1F= 96500C]
- A. 6.40g
- B. 0.67g
- C. 0.64g
- D. 0.45g
Which of the following factors characterize members of the same homologous series?
- A. The chemical property change gradually throughout the series
- B. physical properties of members are similar
- C. They have the same molecular formular
- D. Successive members undergo changes in molecular formula by \(CH_{2}\)
Consider the following reaction equation: \(2NO_{(g)} + O_{{2}{(g)}} \to 2NO_{{2}{(g)}}\). What is the change in the oxidation number of nitrogen?
- A. +2 to 0
- B. +2 to -2
- C. +2 to +4
- D. +4 to +2
The common feature of reactions at the anode is that
- A. electrons are consumed
- B. ions are reduced
- C. oxidation is involved
- D. the electrode dissolves


