(a) Write the molecular formula of X.
(i) What type of reaction is represented by the equation?
(ii) Consider the following reaction equation: C\(_{12}H_2\) \(\to\) X + C\(_8\)H\(_{18}\)
(iii) Draw the structure of two isomers of X.
(iv) Name the isomers drawn in (a)(iii).
(v) Write a balanced equation for the reaction between X and hydrogen.
(b) Describe one test for fats.
(c) Sulphur (IV) oxide is converted to tetraoxosulphate (VI) acid according to the following equation: 2SO\(_{2(g)}\) + O\(_{2(g)}\) + 2H\(_2\)O\(_{(l)}\) \(\to\) 2H\(_2\)OSO\(_{4(aq)}\). If 1.5 moles of oxgen reacts with sulphur (IV) oxide, calculate the mass of tetraoxosulphate (VI) acid produced. [H = 1.0; O = 16.0; S = 32.0].
(d) Consider the following neutralization reaction:
CH\(_3\)COOH + NaOH \(\to\) CH\(_3\)COONa + H\(_2\)O; \(\bigtriangleup\)H\(_1\)
CH\(_3\)COOH + NH\(_4\)OH \(\to\) CH\(_3\)COONH\(_4\) + H\(_2\)O; \(\bigtriangleup\)H\(_2\)
NaOH + HCl \(\to\) NaCl + H\(_2\)O \(\bigtriangleup\)H\(_3\)
(i) Arrange the enthalphy changes for the reactions in order of increasing magnitude.
(ii) Explain briefly your order in (d)(i).
(e) Consider the following substances. Cu\(_{(s)}\), BeCl\(_2\), NaH\(_{(s)}\), HF\(_{(s)}\)and CCl\(_{4(l)}\). State the substance(s) which;
(i) can conduct electricity;
(ii) is/are soluble in water.
Explanation
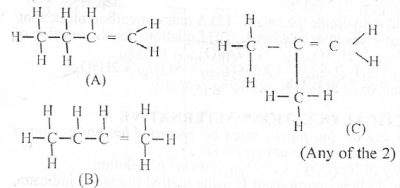
(a)(i) Structure of two isomers of X
(ii) Catalytic cracking
(iii) C\(_4\)H\(_8\) (12 - 8) = 4, (26 - 18) = 8
(iv) Name of Isomers drawn in a(iii) are: (A) But - 1- ene (B) But - 2 - ene (C) 2-methyl propene
(v) Balanced equation for the reaction between X and hydrogen.
C\(_4\)H\(_8\) + H\(_2\) ---> C\(_4\)H\(_{10}\)
(b) Test for fats: A small amount of the substance placed on a filter paper then rub or warm or melt on the filter paper. Formation of a translucent spot on the filter paper shows or indicates the presence of fats presence of fat or a small amount of the substance treated with Sudan III reagent/or Add Sudan III to the sample. Observation of a red colour shows the presence of fat.
(c) Molar mass of H\(_2\)SO\(_4\): [(1 x 2) + 32 + (4 x16)]
= 2 + 32 + 64 = 98 — 98g mol\(^{-2}\)
1 mole of O\(_{2(g)}\) --> 2 moles of H\(_2\)SO\(_2\)
1.5 mole of O\(_{2(g)}\) --> (2 x 1.5) moles of H\(_2\)SO\(_4\) 3 moles of H\(_2\)SO\(_4\)
Mass of 3 moles of H\(_2\)SO\(_4\) = 3 x 98 = 294g.
(d) (i) Enthalpy changes for reactions in their increasing magnitude.
\(\bigtriangleup\)H\(_2\) < \(\bigtriangleup\)H\(_1\) < \(\bigtriangleup\)\(_3\) or \(\bigtriangleup\)H\(_2\) \(\bigtriangleup\)H\(_1\) \(\bigtriangleup\)H\(_3\), or \(\bigtriangleup\)H\(_3\) > \(\bigtriangleup\)H\(_1\) > \(\bigtriangleup\)H\(_2\) < lesser than > greater than
(ii) The explanation in the order of arrangement in (d) (i): Neutralization involves the combination of H\(^+\) and OH\(^-\) ions to give a specific amount of energy. The greater the ionization, the greater the energy given off \(\bigtriangleup\)H\(_2\) involves a weak acid and a weak base/weak electrolyte. \(\bigtriangleup\)H\(_1\), involves a stronge base and a weak acid. \(\bigtriangleup\)H\(_3\) involves a strong acid and a strong base.
(e) (i) Cu\(_{(s)}\) can conduct electricity,
(ii) NaH\(_{(g)}\) , BeCl\(_{2(s)}\), HF\(_{(l)}\) are soluble in water.

