The solubility of \(CO_{2}\) in water can be accounted for by
- A. Van der Waal's forces
- B. Ionic attraction
- C. dipole attraction
- D. covalent bonding
The bonding pair of electrons in a Hydrogen Chloride molecule is pulled towards the chlorine atom because
- A. chlorine has a larger atomic size
- B. chlorine has a larger atomic mass
- C. chlorine is more electronegative
- D. there is no bonding orbitals within the hydrogen atom
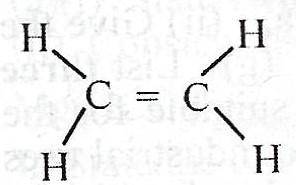
What is the total number of shared pair of electrons in the compound above?
- A. 5
- B. 8
- C. 10
- D. 12
The atomic number of an isotope of hydrogen is equal to its mass number because it
- A. has a totally filled valence shell
- B. has a high charge to mass ratio
- C. does not contain neutrons
- D. exhibits isotopy
Which of the following electron configurations represents the transition element Chromium \(_{24}Cr\)?
- A. \(1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 4s^{2} 3d^{4}\)
- B. \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{6}\)
- C. \(1s^{2}2s^{2}2p^{6}3s^{2}3d^{4} 4s^{1}\)
- D. \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{1}3d^{5}\)
Common Salt (NaCl) is used to preserve foods. Which of the following properties can be used to determine its purity before use?
- A. Solubility in water
- B. Melting point
- C. Relative density
- D. Crystalline nature
Atoms are electrically neutral because they
- A. do not conduct electricity
- B. contain equal number of protons and electrons
- C. are composed of neutrons and electrons
- D. cannot be attracted by electromagnetic field.
What is the relative molecular mass of a compound which has empirical formula \(CH_{2}O\)? [C= 12, H= 1, O= 16]
- A. 42
- B. 45
- C. 126
- D. 180
The law of definite proportions states that
- A. pure samples of the same compound contain the same elements combined in the same proportion by mass
- B. pure samples of substances are in the same proportion by mass
- C. chemical compounds are pure because they contain the same elements
- D. matter can neither be created nor destroyed.
Analysis of a hydrocarbon shows that it contains 0.93g of Carbon per gram of the compound. The mole ratio of carbon to hydrogen in the compound is [H = 1.0, C=12.0]
- A. 1:1
- B. 1:2
- C. 2:1
- D. 2:3
Which of the following statements is true about ionic radius? Ionic radius
- A. increases as nuclear charge increases
- B. decreases as nuclear charge increases
- C. decreases as nuclear charge decreases
- D. remains constant as nuclear charge increases
Which of the following methods can be used to seperate blood cells from plasma?
- A. Centrifugation
- B. Filtration
- C. Chromatography
- D. Distillation
The change in the oxidation state of iron in the reaction represented by the equation: \(2FeCl_{3} + H_{2}S \to 2FeCl_{2} + 2HCl + S\) is
- A. +2 to +3
- B. 0 to +2
- C. +3 to +2
- D. +3 to 0
The empirical formula of a compound containing 0.067mol Cu and 0.066mol O is [Cu = 63.5, O = 16]
- A. \(Cu_{2}O\)
- B. \(CuO\)
- C. \(CuO_{2}\)
- D. \(CuO_{4}\)
When zinc is added to AgNO\(_{3}\) solution, crystals of silver forms on the zinc surface. This indicates that zinc is
- A. oxidised
- B. reduced
- C. decomposed
- D. dissociated
The strength of metallic bonds depends on the
- A. charge density of the atoms
- B. ductility of the metal
- C. number of valence electrons
- D. total number of electrons in the atom
How many atoms are contained in 0.2moles of nitrogen? \([N_{A} = 6.02 \times 10^{23}]\)
- A. \(1.20 \times 10^{23}\)
- B. \(2.41 \times 10^{23}\)
- C. \(3.62 \times 10^{23}\)
- D. \(4.82 \times 10^{23}\)
When chlorine is passed through a sample of water, the pH of the water sample would be
- A. < 7
- B. > 7
- C. = 7
- D. 0
One of the criteria for confirming the purity of benzene is to determine its
- A. heat capacity
- B. boiling point
- C. mass
- D. colour
Which of the following CANNOT be an empirical formula?
- A. \(CH\)
- B. \(CH_{2}\)
- C. \(P_{2}O_{5}\)
- D. \(N_{2}O_{4}\)
\(Cu\) and \(HNO_{3}\) are not suitable for preparing hydrogen gas because of their
- A. reactivity and oxidation respectively
- B. conductivity and corrosiveness respectively
- C. melting point and reduction respectively
- D. electronegativity and solubility respectively


