Consider the following reaction equation: \(2HCl + Ca(OH)_{2} \to CaCl_{2} + H_{2}O\). What is the volume of 0.1\(moldm^{-3}\) HCl that would completely neutralize 25\(cm^{3}\) of 0.3\(moldm^{-3}\) Ca(OH)\(_{2}\)?
- A. 150\(cm^{3}\)
- B. 75\(cm^{3}\)
- C. 30\(cm^{3}\)
- D. 25\(cm^{3}\)
Which of the following statements best explains the difference between a gas and a vapour?
- A. Unlike gases, vapours are liquids at room temperature
- B. Unlike gases, vapour can easily be condensed into liquids
- C. Unlike gases, vapour is readily converted into solids
- D. Vapours are generally denser than gases
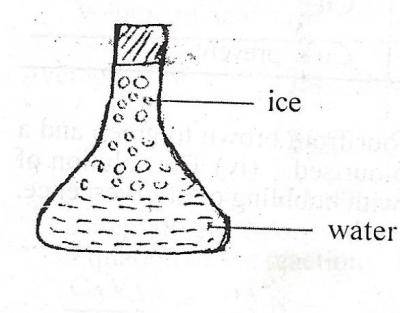
The diagram above illustrates a conical flask containing water and ice. Which of the following is correct about the diagram?
- A. The water is at lower temperature than the ice
- B. Energy is absorbed when the ice changes to water
- C. Energy is released when the ice changes to water
- D. The water molecules vibrate about a fixed point
The position of equilibrium in a reversible reaction is affected by
- A. particle size of the reactants
- B. vigorous stirring of the reaction mixture
- C. presence of a catalyst
- D. change in concentration of the reactants
The following factors would contribute to environmental pollution except
- A. production of ammonia
- B. manufacture of cement
- C. photosynthesis
- D. combustion
The most suitable substance for putting out petrol fire is
- A. water
- B. carbon(IV) oxide
- C. fire blanket
- D. sand
The number of Hydrogen ions in 1.0\(dm^{3}\) of 0.02\(moldm^{-3}\) tetraoxosulphate(VI) acid is \([N_{A} = 6.02 \times 10^{23}]\)
- A. \(1.2 \times 10^{22}\)
- B. \(1.2 \times 10^{23}\)
- C. \(2.4 \times 10^{22}\)
- D. \(2.4 \times 10^{23}\)
Stainless steel is an alloy comprising of
- A. Fe and C
- B. Fe and Ni
- C. Fe, C and Ni
- D. Fe, C and Al
The valence electrons of \(_{12}Mg\) are in the
- A. 3s orbital
- B. 2p\(_{x}\)
- C. 2s orbital
- D. 1s orbital
The preferential discharge of ions during electrolysis is influenced by the
- A. mechanism of electrolysis
- B. electrolytic reactions
- C. nature of the electrode
- D. type of electrolytic cell
When substance X was added to a solution of bromine water, the solution became colourless. X is likely to be
- A. propane
- B. propanoic acid
- C. propyne
- D. propanol
When a salt is added to its saturated solution, the salt
- A. dissolves and the solution becomes super saturated
- B. dissolves and the solution becomes unsaturated
- C. precipitates and the solution remains unchanged
- D. dissolves and crystals are formed
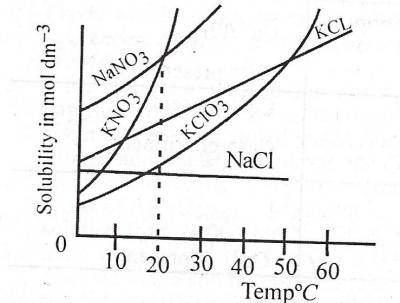
At what temperature does the solubility of \(KNO_{3}\) equal that of \(NaNO_{3}\)?
- A. 0°C
- B. 20°C
- C. 30°C
- D. 40°C
Pure water can be made to boil at a temperature lower than 100°C by
- A. reducing its quantity
- B. decreasing the external pressure
- C. distilling it
- D. increasing the external pressure
What is the mass of solute in 500\(cm^{3}\) of 0.005\(moldm^{-3}\) \(H_{2}SO_{4}\)? ( H = 1, S = 32.0, O=16.0)
- A. 0.490g
- B. 0.049g
- C. 0.245g
- D. 0.0245g
Consider the following equilibrium reaction: \(2AB_{{2}{(g)}} + B_{{2}{(g)}} \to 2AB_{{3}{(g)}}\). \(\Delta H= -X kJmol^{-1}\). The backward reaction will be favored by
- A. a decrease in pressure
- B. an increase in pressure
- C. a decrease in temperature
- D. an introduction of a positive catalyst
Ethene is produced from ethanol by
- A. decomposition
- B. hydrolysis
- C. ozonolysis
- D. dehydration
Consider the following reaction equation: \(Br_{2} + 2KI \to 2KBr + I_{2}\). Bromine is acting as
- A. an oxidizing agent
- B. a reducing agent
- C. an acid
- D. a base
An organic compound has the empirical formula \(CH_{2}\). If its molar mass is 42\(gmol^{-1}\), what is its molecular formula? (C = 12.0, H = 1.0)
- A. \(C_{2}H_{4}\)
- B. \(C_{3}H_{4}\)
- C. \(C_{3}H_{6}\)
- D. \(C_{4}H_{8}\)
In which of the following compounds does hydrogen form ionic compounds?
- A. CH\(_{4}\)
- B. HCl
- C. NH\(_{3}\)
- D. NaH
A reaction is endothermic if the
- A. reaction vessel feels cool during the reaction
- B. enthalpy change is negative
- C. bond forming energy exceeds bond breaking energy
- D. heat of formation of reactants exceeds heat of formation of products


