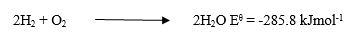(a) A compound of carbon, hydrogen and chlorine contains 0.48 g of carbon, 0.08 g of hydrogen and 1.42 g of chlorine.
(i) Determine the empirical formula of the compound.
(ii) If the molar mass of the compound is 99, calculate the molecular formula of the compound.
[H = 1.0, C = 12.0, Cl = 35.5]
(b) State three properties of NaCl (s) which shows that it is ionic.
(c) Consider the following reaction equation:
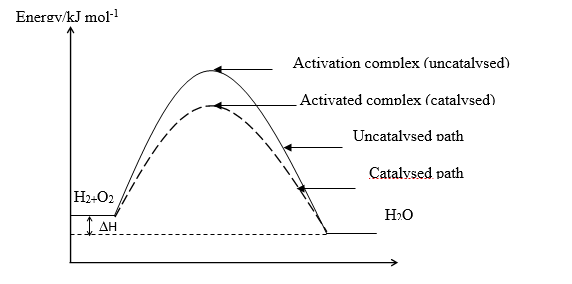
(i) On the same diagram, sketch and label a reaction profile for a catalysed and uncatalysed reaction between H\(_2\) and O\(_2\).
(ii) Indicate the possible positions of the activated complexes for the reaction profiles in (c)(i).
(d) The petrochemical industry produces addition polymers using one of the fractions obtained from crude oil.
(i) Name the fraction used as a raw material for the process.
(ii) What process is used to obtain the fraction from crude oil?
(iii) Name two gaseous hydrocarbons that can be used in making polymers.
(iv) Describe briefly how these hydrocarbons can be obtained.
(v) Name the polymer produced from one of the hydrocarbons named in (d)(iii).
Explanation
(a)(i) ratio of atoms
C = \(\frac{0.48}{12}\) = 0.04, H= \(\frac{0.08}{1}\) = 0.08, Cl = \(\frac{1.42}{35.5}\) = 0.04
Divide by the smallest number
\(\frac{0.04}{0.04}\): \(\frac{0.08}{0.04}\) : \(\frac{0.04}{0.04}\)
1 : 2 : 1
Empirical formula = CH\(_2\)Cl
(ii) Molecular formula = (Empirical formula)n
99 = (CH\(_2\)Cl)n
99 = (12 + (2 x 1) + 35.5)n
99 = 49.5n
n = \(\frac{99}{49.5}\)
= 2
molecular formular = C\(_2\)H\(_4\)Cl\(_2\) / (CH\(_2\)Cl)\(_2\)
(b)
- in its molten form / solution conducts electricity
- has strong electrostatic forces
- has high melting / boiling points
- is highly soluble in water
(c) (i) & (ii)
Reaction co-ordinate
(d) (i) Naphtha Accept kerosene / Diesel
(ii) Fractional distillation
(iii) - Ethene
- Propene
(iv) Catalytic cracking whereby long-chain hydrocarbons (in the naphtha fraction) are broken down into small organic molecules
Conditions are 600\(^o\) C and Al\(_2\)O\(_2\)\(_{(s)}\) / SiO\(_2\)\(_{(s)}\) as catalyst
(v)
- Polyethene
- Polypropane