Which of the following species has the largest ionic radius
- A. S\(^{2-}\)
- B. K\(^{+}\)
- C. CI\(^{-}\)
- D. Ca\(^{2+}\)
What is the empirical formula of a hydrocarbon containing 0.160 moles of carbon and 0.640 moles of hydrogen
- A. CH\(_{2}\)
- B. CH\(_{3}\)
- C. CH\(_{4}\)
- D. C\(_{2}\)H\(_{4}\)
What is the concentration of a solution which contains 0.28g of potassium hydroxide in 100 cm\(^{3}\) of solution [KOH = 56]
- A. 0.01 mol dm\(^{-3}\)
- B. 0.05 mol dm\(^{-3}\)
- C. 0.10 mol dm\(^{-3}\)
- D. 0.50 mol dm\(^{-3}\)
Student X titrated 25 cm\(^{3}\) of Na\(_{2}\)CO\(^{3}\) with 0.1 mol dm\(^{-3}\) HCI, using methyl orange as indicator. Student Y carried out the same exercise but used phenolphthalein as an indicator. Which of the following statements about the titration is true?
- A. hydrogen chloride gas was released in the reactions in both titrations
- B. the titer values obtained from the titration are equal
- C. the tirer value obtained by X is twice that of Y
- D. the titer value obtained by Y is twice that of X
The 25°C evaporation of a 100 cm\(^{3}\) solution of K\(_{2}\)CO\(_{3}\) to dryness gave 14g of the salt. What is the solubility of K\(_{2}\)CO\(_{3}\) at 25°C? [K\(_{2}\)CO\(_{3}\) = 138]
- A. 0.01 mol dm\(^{-3}\)
- B. 0.101 mol dm\(^{-3}\)
- C. 1.01 mol dm \(^{-3}\)
- D. 10.0 mol dm\(^{-3}\)
The Bohr model of the atom proposed the existence of?
- A. the nucleus
- B. electron shells
- C. nucleons
- D. neutrons
How many coulombs of electricity would liberate 1.08g of Ag from a solution of silver salt?
[Ag = 108.0; 1F = 96500 C]
- A. 96500 C
- B. 9650 C
- C. 965 C
- D. 9.65 C
The reactivity of fluorine is high because of
- A. its high electronegativity
- B. the small size of the fluorine atom
- C. the availability of d-orbitals
- D. the strong F-F bond
Consider the following diagram and use it to answer the question below

Which of the following half reaction equations represent the reaction at the cathode
- A. A\(^{3+_{(aq)}}\)+3e\(^{-}\) \(\rightarrow\) A\(_{(s)}\)
- B. B\(^{2+_{(aq)}}\)+2e\(^{-}\) \(\rightarrow\) B\(_{(s)}\)
- C. A\(_{(s)}\) \(\rightarrow\) \(^{3+_{(aq)}}\)+3e\(^{-}\)
- D. B\(_{(s)}\) \(\rightarrow\) B\(^{2+_{(aq)}}\) + 2e\(^{-}\)
Consider the following diagram and use it to answer the question below

Which of the following cell notations represent the diagram
- A. B\(^{2}\)+/B/ /A/A\(^{3+}\)
- B. A\(^{3+}\) /A/ /B/B\(^{2+}\)
- C. B/B\(^{2+}\)/ /A/\(^{3+}\)
- D. A/A\(^{3+}\)/ /B\(^{2+}\)/B
Which of the following statements about Group VII elements is correct?
- A. they are present in the same physical state
- B. they are strong reducing agents
- C. their reactivity decreases down the group
- D. they exist as monoatomic molecules
Elements with high ionization energies would——-
- A. lose electrons easily
- B. have large atomic radii
- C. have high effective nuclear charges
- D. have low atomic numbers
The collision between ideal gas molecules are considered to be perfectly elastic because
- A. they collide without losing energy
- B. they move randomly in a straight line
- C. their average kinetic energy is variable
- D. the distance between them is large compared to their size
Under which conditions of pressure (p) and temperature (T) would the volume of an inflated ballon increase? When
- A. P and T are increased
- B. both T and P are decreased
- C. T is increased and P is decreased
- D. T is decreased and P is increased
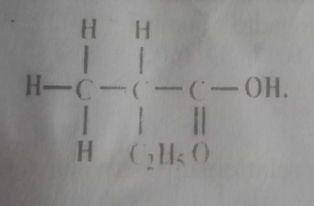
Consider the structure above
How many carbon atoms does the parent chain contain?
- A. 5
- B. 4
- C. 3
- D. 2
When CuSO\(_{4(aq)}\) is added to Pb(NO\(_{3}\))\(_{2(aq)}\)——–
- A. there would be no visible change
- B. a blue precipitate would be formed
- C. the resulting solution would become colourless
- D. a white precipitate would be formed
What would be observed when aqueous ammonia is added in drops and then in excess to a solution of copper(II) ions?
- A. blue precipitate is formed which is soluble in excess ammonia
- B. brick red precipitate is produced which is insoluble in excess ammonia
- C. white precipitate is formed which is excess in ammonia
- D. green precipitate is formed which is insoluble in excess ammonia
The basic property of salts used as drying agents is by?
- A. efforescence
- B. high melting point
- C. hygroscopy
- D. low solubility
What number of moles of oxygen would exert a pressure of 10 atm at 320 K in an 8.2 dm\(^{2}\) cylinder?
[R = 0.082 atm dm\(^{3}\) mol\(^{-1}\) K\(^{-1}\)]
- A. 0.32
- B. 1.56
- C. 3.13
- D. 31.25
The most common method of preparing insoluble salts is by
- A. filtration
- B. decomposition
- C. neutralization
- D. double decomposition
A molecular of phosphorus is
- A. diatomic
- B. triatomic
- C. tetraatomic
- D. monoatomic


