Burette readings (initial and final) must be given to two decimal places. Volume or pipete used must also be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A is a solution containing 12.0g dm\(^{-3}\) NaHSO\(_4\) NaHSO\(_4\) P is a solution containing NaOH
(a) Put A into the burette and titrate it against 20.0cm\(^3\) or 25.0m\(^3\) portions of B using methyl orange as an indicator. Repeat the titration to obtain consistent titres. Tabulate your readings and calculate the average volume A used. The equation for the reaction involved in the titration is ;
NaHSO\(_{4(aq)}\) + NaOH\(_{(aq)}\) \(\to\) Na\(_2\)SO\(_{4(aq)}\) + H\(_2\)O\(_{(l)}\)
[H 1.00, O = 16.0; Na = 23.0, S = 32.0]
(b) From your results and the information provided above calculate the:
(i) concentration of A in mol dm\(^{-3}\)
(ii) concentration of B in mol dm\(^{-3}\)
(iii) mass of Na\(^+\) formed in solution during the titration.
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C and D are inorganic salts. X is a solution of an inorganic compound. Carry out the following exercises on C, D and X. Record your observations and identity any gas(es) involved. State the conclusion drawn from the result or each test.
(a)(i) Add about 5 cm\(_3\) of distilled water to C in a test tube and shake thoroughly, Divide the resulting solution into two portions.
(ii) Put about 2cm\(^3\) of X into a separate test tube and add the first portion of solution in (a)(i) To the second portion of the resulting solution in (a)(i), add few drops of BaCl\(_{2(aq)}\) followed by excess dil. HCI.
(b) Add about 10 cm of distilled water to D in a boiling tube and shake thoroughly.
(i) To about 2 cm\(^3\) portion of the resulting solution in a test tube, add NaOH\(_{(aq)}\) in drops and then in excess
(ii) To another 2 cm\(^3\) portion of the resulting solution add few drops of K\(_2\)CrO\(_{4(aq)}\)
(a) An aqueous solution of salt Q was added to excess Sodium trioxocarbonate (V) solution in a test tube. There was effervescence, the test tube became warm and a white precipitate was observed. State three inferences that could be drawn from these observations.
(b) Consider the following salts: NH\(_4\)CI; PbSO\(_4\); NaHCO\(_3\), Cu(NO\(_3\))\(_2\) and ZnCO\(_3\). Select from the list, the salt(s) which;
(i) do not/does not readily dissolve in water;
(i) produce(s) effervescence with dilute mineral acids;
(ii) decompose(s) on heating
(iv) dissolves(s) in water to form an alkaline solution;
(v) sublime(s) on heating.
Burette readings (initial and final) must be given to two decimal places. Volume of pipefte used must also be recored but no account of expeririental procedure is required. All calculations must be done in your answer book.
A is 0.100 mol dm\(^{-3}\) solution of an acid. B is a solution of KOH containing 2.8 g per 500 cm’\(^3\)
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as an indicator. Repeat the titration to obtain consistent titres. Tabulate your readings and calculate the average volume of A used.
(b) From your results and the information provided above, calculate the:
(i) number of moles of acid in the average titre;
(i) number of moles of KOH in the volume of B pipetted;
(ii) mole ratio of acid to base in the reaction. [H = 1.00, O = 16.0, K = 39.0]
Credit will be given for strict adherence to the instructions, for observations precisely record and for accurate inferences. All tests, observations and inferences must be clearly entered in your answer book in ink, at the time they are made.
C is a mixture of two salts. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion drawn from the result of each fest
(a) Put all of C into a boiling tube and add about 5cm\(^3\) of distilled water. Stir thoroughly and filter. Keep both the residue and the filtrate.
(b) To about 2 cm\(^3\) of the filtrate, add few drops of Pb(NO\(_3\))\(_{(aq)}\). Boil the mixture and then allow to cool.
(c)(i) Put the residue in a test tube and add dilute HNO\(_{3}\). Shake the mixture and divide the solution into two portions
(ii) to the first portion from (c)(i), add NaOH\(_{(aq)}\) in drops acid then in excess.
(iii) To the second portion from (c)(ii), add aqueous ammonia in drops and then in excess
(a) A colourless gas P was given off when dilute tetraoxosulphate (VI) acid was added to zinc salt Q. On bubbling the gas through lime water, a white precipitate R was formed. Identify P, Q and R.
(b) Name a suitable apparatus that could be used to perform each of the following activities in the laboratory
(i) storage of dilute silver trioxonitrate (V):
(ii) heating copper metal;
(iii) separation of a mixture of water and Kerosene. Give one reason for each of your answers in (b)
Burette readings (initial and final) must be given to two decimal places. Volume of pipette used must also be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A solution containing 6.22 g of an acid H\(_2\)Y per dm\(^3\)
B contains 3.90 g of NaOH per dm\(^3\) of solution.
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as indicator. Repeat the titration to obtain consistent titres. Tabulate your burette readings and calculate the average volume of acid A used. The equation for the reaction involved in the titration is:
H\(_2\)\(_{(aq)}\) + 2NaOH\(_({aq})\) \(\to\) NaY\(_{(aq)}\) + 2H\(_2\)O\(_{(l)}\)
[H = 1.00; O = 16.0; Na = 23.0]
(b) From your results and the information provided above, calculate the:
(i) The concentration of B in moldm\(^{-3}\)
(ii) concentration of A in moldm\(^3\)
(ii) molar mass of H\(_2\)Y.
(c) State whether the pH of each of the following solutions is lower than 7, greater than 7 or equal to 7. The
(I) solution A before titration
(ii) solution B before titration
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C and D are two aqueous solutions. Carry out the following exercises on C and D. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a)(i) To about 2 cm\(^3\) portion of C, add NaOH\(_{(aq)}\) In drops until in excess. Warm the mixture
(ii) To another 2 cm\(^3\) portion of C, add HCl\(_{(aq)}\) followed by BaCl\(_{2(aq)}\)
(b)(i) (ii) To another 2 cm\(^3\) portion of D, add add NH\(_{3(aq)}\) drops and then in excess.
(ii) To about 2 cm\(^3\) portion of D, add AgNO\(_{3(aq)}\) followed by HNO\(_{3(aq)}\)
(a) Explain briefly the observations in each of the following processes:
(i) when carbon (IV) oxide is bubbled through lime water, It turns milky but the milkiness disappears when the gas is bubbled for a long time.
(ii) A precipitate of calcium hydroxide is insoluble in excess sodium hydroxide solution whereas that of lead (ii) hydroxide is soluble
(b)(i) What is a primary standard solution?
(ii) Calculate the mass of sodium trioxocarbonate (V) required to prepare 250 cm\(^3\) of 0.15gmoldm\(^3\) solution hydroxide is soluble. [Na = 23.0; O = 16.0; C = 12.0]
(c) Name one gas that can be collected by
(i) upward displacement of air
(ii) downward displacement of air
Burette readings (initials and final) must be given to two decimal places. Volume of pipette used must also be recorded but no account experimental procedure is required. Al calculations must be done in your answer book. A solution containing 0.05moldm\(^3\) H\(_2\)SO\(_4\). B is a solution containing 1.4g per 250cm\(^3\) .XOH
(a) Put A into the burette and titrate it against 20.0cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange indicator. Repeat the titration to obtain consistent titres. Tabulate your results and calculate the average volume of A used. The equation for the reaction involved in the titration is; H\(_2\)SO\(_{4(aq)}\) + 2XOH\(_{(aq)}\) \(\to\) X\(_2\)SO\(_{4(aq)}\) + 2H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided above, calculate the;
(i) concentration of B in moldm\(^{-3}\)
(ii) molar mass XOH
(iii) relative atomic mass of X. [H = 1.00; O = 16.0 S =32.0]
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. AIl tests. observations and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is a double salt. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion drawn from the result of each test.
a) Put all of C into a test tube. Add about 5cm\(^3\) of distilled water, stir and test with litmus paper. Divide the Solution into two portions
(b) To the first portion, add sodium hydroxide solution in drops and then in excess. Heat the resulting mixture and keep it for minutes.
(c) To the second portion, add few drops of Bacl\(_{2(aq)}\) followed by excess dilute hydrochloric acid
(a)i) How would an aqueous solution of iron (II) tetraoxosulphate (VI) be converted into an aqueous solution of magnesium tetraoxosulphate (VI)?
(ii) Write a balanced equation for the reaction in (a)(i) above
(b)(i) Why are some compounds recrystallized after preparation?
(ii) Outline the steps in recrystallization
(C)(i) Name two gases that can cause color changes in an acidified solution of potassium heptaoxodichromate (VI)
(ii) State the color change expected in (c)i) above
Burette readings (initial and final) must be given to two decimal places. Volume of pipette used must also be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A is a solution of hydrochloric acid. B is a solution containing 2.45g of anhydrous sodium trioxocarbonate (IV) in 250g of solution.
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portion of B using methyl orange as an indicator. Repeat the exercise to obtain consistent titres. Tabulate your burette reading and calculate the average volume of A used. The equation for the reaction involved in the titration is Na\(_2\)CO\(_{3(aq)}\) + 2HCI\(_{(aq)}\) + H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided, calculate the:
(i) concentration of B in moldm\(^{-3}\)
(ii) concentration of A in moldm\(^{-3}\)
(iii) concentration of A in gdm\(^{-3}\)
(iv) volume of the gas evolved in the reaction at s.t.p.
[H = 1.00; C: 12.0; O = 16.0; Na = 23.0; Na = 23.0; Cl = 35.5; Molar Volume = 22.4 dm\(^3\)mol\(^{-3}\)]
Credit will be given for strict adherence to the instructions, for observations, precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C contains two cations and two anions. Perform the following exercises on C. Record your observations and identify any gas (es) evolved. State the conclusion you draw from the result of each test.
(a) Dissolve all of C in about 10 cm\(^3\) of distilled water. Stir the resulting solution thoroughly.
(i) To about 2 cm\(^3\) of the solution, add few drops of AgNO\(_3\) solution, followed by HNO\(_{3(aq)}\). To the mixture, add excess NH\(_{3(aq)}\)
(ii) To another 2 cm\(^3\) portion of the solution, add dil. HCl followed by BaCl\(_2\) solution.
(iii) To another 2 cm\(^3\) portion of the solution, add NaOH\(_{3(aq)}\) dropwise and then in excess. Warm the mixture.
(iv) To another 2 cm\(^3\) portion of the solution, add NH\(_{3(aq)}\) dropwise and then in excess.
Consider the following experimental set-up.

(i) Identify by name: P; Q; R; S and T.
(ii) State the method of collection of gas, S.
(iii) What is the function of R in the experimental set-up?
(iv) Write the balanced equation of the reaction for the preparation of gas S.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account experimental procedure is required. All calculations must be done in your answer booklet.
A is 0.100 mol dm\(^{-3}\) HNO\(_3\). B is a solution containing 2.50 g of a mixture of Na\(_2\)CO\(_3\) and Na\(_2\)SO\(_4\) in 250 cm\(^3\) of solution.
(a) Put into the burette and titrate it against 20.0 cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange as indicator. Repeat the exercise to obtain consistent titre values. Tabulate your readings and calculate the average volume of acid A used. The equation of reaction is Na\(_2\)CO\(_{3(aq)}\) + 2HNO\(_{3(aq)}\) \(\to\) NaNO\(_{3(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
(b) From your results and the information provided, calculate the:
(i) concentration of B in moldm\(^{-3}\);
(ii) concentration of Na\(_2\)CO\(_3\) in B in gdm\(^{-3}\);
(iii) percentage of Na\(_2\)CO\(_3\), in the mixture. [Na\(_2\)CO\(_3\) = 106]
Credit will be given for strict adherence to the instructions, for observations precisely recorded and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C and D are inorganic salts. E is a solution of an organic compound. Carry out the following exercises on C, D and E. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) To all of C, add about 2 cm\(^3\) of distilled water in a boiling tube. Shake to dissolve and test the solution with litmus paper.
(b)i) Test E with litmus paper.
(ii) Add all of E to the solution obtained in (a).
(c)(i) Add about 5 cm\(^3\) of distilled water to D in a test tube and shake thoroughly. Divide the resulting solution into two portions.
(ii) To the first portion, add few drops of BaCl\(_{2(aq)}\) followed by excess dil. HCl.
(iii) To the second portion, add HNO\(_{3(aq)}\) in drops and then in excess.
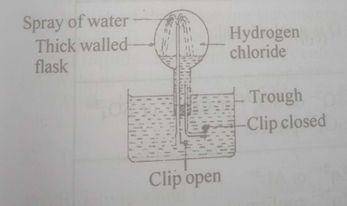
(a) Consider the following diagram ABOVE.
(i) Give the name of the experiment.
(ii) What does the experiment demonstrate?
(iii) Name one gas that could be used in place of HCl gas.
(iv) What colour could be observed in the flask during the spray of water?
(v) Could the gases used in the experiment be collected over water?
(vi) Explain briefly your answer in (a)(v).
(b) A solid substance U when strongly heated decomposes to give a white solid V and carbon (IV) oxide. When water is added to V, W is produced W can be used to test for carbon (IV) Oxide. Identify U, V and W.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet
A solution of 0.050 moldm\(^3\) H\(_2\)C\(_2\)O\(_4\) (ethanedioic acid). B is a solution of KMnO\(_4\), (potassium tetraoxomanganate (VII), of unknown concentration.
(a) Put B into the burette. Pipette 20.0 cm\(^3\) or 25.0 cm\(^3\) of A into a Conical flask and add about 10.0 cm\(^3\) of dilute H\(_2\)SO\(_4\), Heat the mixture to about 40°C – 50°C and titrate it while still hot with B. Repeat the titration to obtain consistent titre values. Tabulate your results and calculate the average volume of B used. The equation of reaction is;
2MnO\(_{4(aq)}^-\) + 5C\(_2\)O\(_{4(aq)}^{2-}\) + 16H\(^+_{(aq)}\) \(\to\) 2MnH\(^{2+}_{(aq)}\) + 8H\(_2\)O\(_{(l)}\) + 10CO\(_{2(g)}\)
(b) From your results and the information provided, calculate the:
(i) concentration of MnO\(_2^-\) in B in moldm\(^{-1}\)
(ii) concentration of KMnO\(_4^-\) in B in gdm\(^{-3}\)
(iii) volume of CO\(_2\) evolved at s.t.p when 25.0 cm\(^3\) of H\(_2\)C\(_2\)O\(_4\) reacted completely. [0 = 16.0, K= 39.0, Mn = 55.0, Molar volume of gas at s.t.p.= 22.4 dm\(^3\) mol\(^{-1}\)]
Credit will be given for strict adherence to the instructions. for observations precisely recorded and jor accurate inferences. All tests, observations and inferences must be clearly entered in your answer book in ink, at the time they are made
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet
C is a mıxture of two salts, containing one cation and two anions. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) Put all of C in a beaker and add about 10 cm\(^3\) of distilled water. Stir well and filter. Keep the filtrate and the residue.
(b) To about 2 cm\(^3\) of the filtrate, add few drops of AgNO\(_{(aq)}\), followed by HNO\(_{3(g)}\). Add excess NH\(_{3(aq)}\) to the resulting mixture.
(c)(i) Put all of the residue into a clean test tube and add about 5 cm of HNO\(_{3(aq)}\)
(ii) To about 2 cm\(^3\) of the solution from 2(c)(i), add NaOH\(_{(aq)}\) in drops and then in excess
(iii) To another 2 cm\(^3\) of the solution from 2(c)(i), add NH\(_{3(aq)}\) in drops and then in excess.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet
(a) What difference in physical properties enable the separation of mixtures by:
(i) simple distillation,
(ii) paper chromatography;
(iii) fractional distillation.
(b) Give a reason for each of the following practices during titration in the laboratory.
(i) White tile is placed under the conical flask.
(ii) Burette readings are always recorded to two decimal places.
(iii) Calculate the volume of 2.5 moldm\(^{-3}\) stock HČI required to prepare 500 cm\(^3\) of 0.20 moldm HCI.

