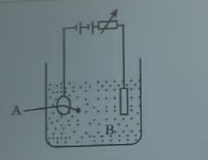
Find the amount of current required to deposit 0.02kg of metal in a given electrolysis for 120 seconds. [electro chemical equivalent of the metal = 1.3 x 10\(^{-7}\)kgC\(^{-1}\)]
The correct answer is: C
Explanation
mass of metal deposited during electrolysis = Zit
Given: mass = 0.02kg, i, t = 120s and Z = 1.3 x 10\(^{-7}\)kgC\(^{-1}\)]
i = \(\frac{\text{m}}{\text{Zt}}\)
i = \(\frac{0.02}{1.3 \times 10^{-7} \times 120}\) = 1282.05 ≈ 1.3 x 10\(^3\)A
There is an explanation video available .


