(a) Define the boiling point of a liquid.
(b) With the aid of a sketch diagram, describe an experiment to determine the boiling point of a small quantity of a Iiquid
(c) A piece of copper of mass 300 g at a temperature of 950°C is quickly transferred into a vessel of negligible thermal capacity containing 250 g of water at 25°C. If the final steady temperature of the mixture is 100°C, calculate the mass of water that will boil away. Especific heat capacity of copper = 4.0 x 10\(^2\) J kg\(^{-1}\)K\(^{-1}\) specific heat capacity of water = 4.2 x 10\(^{3}\) J kg\(^{-1}\)K\(^{-1}\) specific latent heat of vaporization of steam = 2.26 x 10\(^6\)J kg\(^{-1}\)
Explanation
(a) Boiling point of a liquid is the temperature at which the saturated vapour pressure of the liquid is equal to the external atmospheric pressure.
(b)
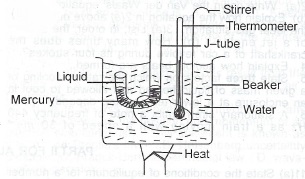
Add and trap a few drops of the liquid in the closed limb of the J-tube above the mercury. Insert into a beaker of water the J-tube, stirrer and thermometer. Heat the beaker and content and stir continuously. Then read and record the temperature at which the mercury levels in the two limbs are the same. Heat the beaker a little further and remove heat source. Also read and record the temperature at which the mercury levels are once again the same. The average of the two temperature values is the boiling point of the liquid.
(c) Heat lost by copper = Heat gained by water
0.3 x 400 (950 - 100) = 0.25 x 4200 x (100 - 25) + 2.26 x 10\(^6\) x m 102000
= 78750 + 2.26 x 10\(^6\)m
m = \(\frac{102000 — 78750}{2.26 \times 10^6}\)
= 0.0102876 kg or 10.3g

