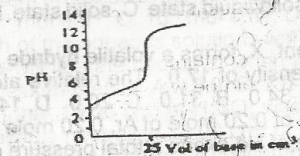
The option above shows the PH changes for the titration of a
- A. strong acid versus strong base
- B. weak acid versus strong base
- C. strong acid versus weak base
- D. weak acid versus weak base
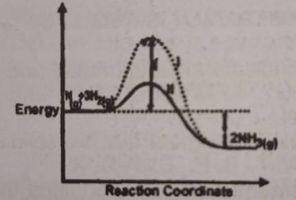
It can be deduced that the rate of the reaction?
- A. for bath l is higher than path ll
- B. for path ll is higher than path l
- C. is the same for both paths at all temperatures
- D. depends on the values of both x and y at all pressures
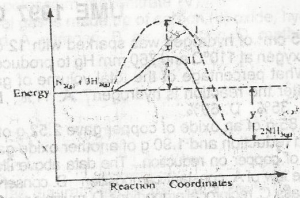
Use the diagram above to answer this question. The activation energy of the uncatalysed reaction is
- A. X
- B. X + Y
- C. X - Y
- D. Y
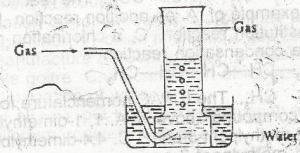
The arrangement above can be used for the collection of
- A. sulphur (IV) oxide
- B. ammonia
- C. hydrogen chloride
- D. nitrogen

USE THE SECTION OF THE PERIODIC TABLE ABOVE TO ANSWER THIS QUESTION.Which letter represents a non-metal that is a solid at room temperature?
- A. T
- B. R
- C. J
- D. X

USE THE SECTION OF THE PERIODIC TABLE ABOVE TO ANSWER THIS QUESTION.Which of the indicate an alkali metal and a noble gas respectively?
- A. M and E
- B. G and E
- C. R and L
- D. G and L
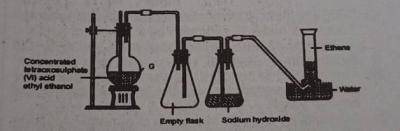
Use the diagram above to answer this question. The reaction taking place in flask G is known as?
- A. hydrolysis
- B. double decomposition
- C. dehydration
- D. pyrolysis
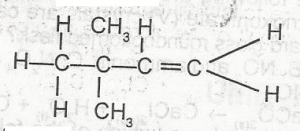
The IUPAC name of the compound above is
- A. 2,2-dimethyl but -1-yne
- B. 2,2-dmethyl but -1-ene
- C. 3,3-dimethyl but -1-ene
- D. 3,3-dimethyl but-1-yne
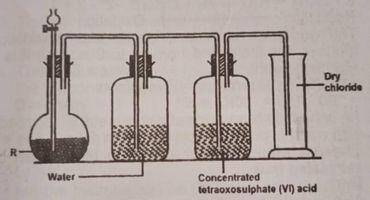
In the diagram above, R is a mixture of?
- A. potassium tetraxochlorate (VII) and concentrated H2SO4
- B. potassium trioxochlorate (V) and concentrated H2SO4
- C. potassium tetratoxomanganate (VII) and concentrated HCI
- D. manganese (IV) oxide and concentrated HCI
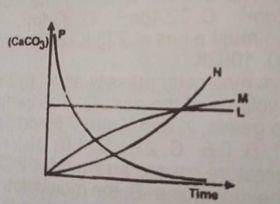
2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from the reaction above, which of the following curves represents the consumption of calcium trioxocarbonate (IV) as dilute HCL is added to it?
- A. L
- B. M
- C. N
- D. P
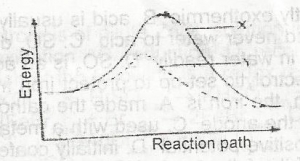
In the diagram above the activation energy is represented by
- A. y - x
- B. x
- C. x - z
- D. y
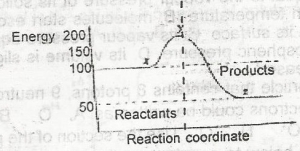
The ∆H for the reaction represented by the energy profile above is
- A. -100 kJ mol--1
- B. + 100 kJ mol-1
- C. +50 kJ mol-1
- D. -50 kJ mol-1
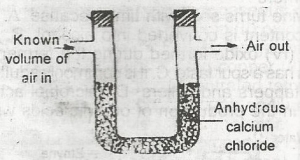
The set-up above would be useful for determining the amount of
- A. oxygen in air
- B. water vapour in air
- C. CO2 in air
- D. arygen in air
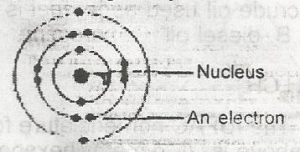
The diagram above represents an atom of
- A. magnesium
- B. helium
- C. chlorine
- D. neon
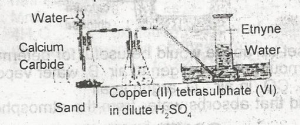
The function of the copper (ll)tetraoxosulphate (IV) in dilute H2SO4 in the figure above is to
- A. dry the gas
- B. absorb phosphine impurity
- C. absorb ethene impurity
- D. from an acetylide with ethyne
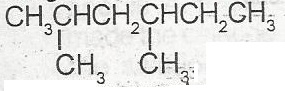
The IUPAC nomenclature for the compound above is
- A. dimethlhexane
- B. 3,5 dimethylhexane
- C. 1.1 dmethyl, 3 methylpentane
- D. 2.4 dimethylhexane

The two functional groups in the above compound are
- A. alcohol and amine
- B. acid and amine
- C. aldehyde and acid
- D. ketone and amine
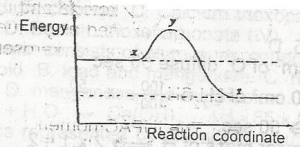
In the diagram above, curve X represents the energy profile for a homogeneous gaseous reaction. Which of the following conditions would produce curve Y for the same reaction?
- A. Increase in temperature
- B. Increase in the concentration of a reactant
- C. Addition of a catalyst
- D. Increase in pressure

Half-cell reaction. From the data above , it can be deduced that the most powerful reducing agent of the four metals is
- A. Cu
- B. Fe
- C. Ba
- D. Zn
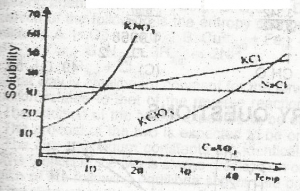
For which salt in the graph above does the solubility increase most rapidly with rise in temperature?
- A. CaSO4
- B. KNO3
- C. NaCl
- D. KCl
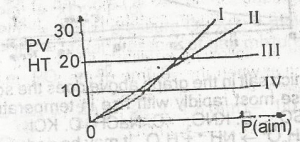
Which of the curves above represents the behaviour of 1 mole of an ideal gas?
- A. l
- B. ll
- C. lll
- D. lV


