Which noble gas is radioactive and is produced as a decay product of uranium and thorium?
- A. Argon
- B. Krypton
- C. Radon
- D. Neon
What happens to the position of equilibrium, if a reversible exothermic reaction is subjected to a decrease in temperature?
- A. The position of equilibrium shifts to the left
- B. The position of equilibrium shifts to the right.
- C. The position of equilibrium remains unchanged
- D. The reaction stops
Hard water is water with high concentrations of dissolved ions, in particular calcium and
- A. magnesium ions
- B. nitrogen ions
- C. phosporus ions
- D. helium ions
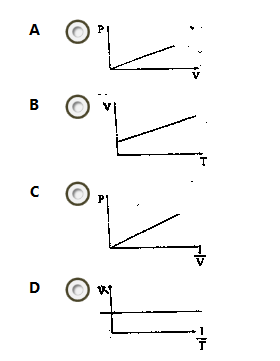
Which of the following sketches is a graphical representation of Boyle’s law?
- A. A
- B. B
- C. C
- D. D
Which of the following scientists formulated the law of conservation of mass?
- A. A. Lavoisier
- B. J. Dalton
- C. R. Boyle
- D. J. Proust
The first definition of an element was made by
- A. J. Dalton.
- B. A. Lavoisier.
- C. R. Boyle.
- D. J. J. Thompson.
Charcoal is used in the decolourization of sugar because of its
- A. absorption property.
- B. amorphous property.
- C. oxidizing property.
- D. adsorption property.
If the molar mass of X(HCO3)\(_{2}\) is 162 g mol\(^{-1}\), determine the relative atomic mass of X.
[H = 1.0, C = 12.0, 0 = 16.0]
- A. 40
- B. 48
- C. 61
- D. 101
Which of the following processes occur during fractional distillation of petroleum?
- A. Condensation and diffusion
- B. Diffusion and evaporation
- C. Diffusion and sublimation
- D. Evaporation and condensation
Which of the following oxides is amphoteric?
- A. Carbon(II) oxide
- B. Nitrogen(IV) oxide
- C. Lead(II) oxide
- D. Calcium oxide
Consider the following table
| Substitute | Meltig point / ºC | Boiling point / ºC |
| P | – 78 | -25 |
| Q | – 8 | 40 |
| R | – 6 | 150 |
| S | 44 | 280 |
Which of the substances is a liquid at room temperature and rapidly evaporates on exposure to air?
- A. P
- B. Q
- C. R
- D. S
Electropositivity of elements across the periodic table normally
- A. remains constant down the group
- B. increases across the period
- C. decreases across the period
- D. decreases down the group
The atom with the electron configuration
1s\(^{2}\)2s\(^{2}\)2p63s\(^{2}\)3p\(^{6}\)3d\(^{10}\)4s\(^{2}\)4p\(^{4}\) is in
- A. period 4, p- block
- B. period 3, p- block
- C. period 4, d- block
- D. period 3, d- block
Which of the following statements about atoms of a metal is correct? They
- A. readily accept electrons
- B. are soft
- C. are held together by covalent bond
- D. are held together by a sea of electron cloud
An oxide has the following properties. It
I. is a white powder
II. reacts with HCI.
III. reacts with NaOH
IV. is insoluble in water
The oxide is
- A. alkaline
- B. amphoteric
- C. acidic
- D. neutral
The electron configuration of carbon atom in its excited state is
- A. 1s\(^{2}\)2s\(^{2}\)2px\(^{2}\)2py\(^{0}\)
- B. 1s\(^{2}\)2s\(^{2}\)2px\(^{1}\)2py\(^{1}\)
- C. 1s\(^{2}\)2s\(^{1}\)2px\(^{1}\)2py\(^{1}\)2pz\(^{1}\)
- D. 1s\(^{1}\)2s\(^{2}\)2px\(^{1}\)2py\(^{1}\)
Which of the following properties indicate that an element is a metal. It
I. reacts with oxygen to form an acidic oxide
II. forms ionic chlorides
III. has variable oxidation states
IV. displaces hydrogen from dilute HCl
- A. I and III only
- B. I and II only
- C. II and IV only
- D. I, II, III and IV
Ionization energy increases across the period in the periodic table because
- A. atomic number increases
- B. effective nuclear charge increases
- C. mass number decreases
- D. screening effect decreases
The IUPAC name of the compound CH3CH(CH3)CHCH2 is
- A. 2 - methyl but - 1 - ene
- B. 2 - methyl but - 2 - ene
- C. 3 - methyl but - 1 - ene
- D. 3 - methyl but - 2 - ene
The maximum number of covalent bonds formed by nitrogen is
- A. 1
- B. 2
- C. 3
- D. 4
Which of the following bond types is intermolecular?
- A. Covalent bond
- B. Hydrogen bond
- C. Ionic bond
- D. Metallic bond


