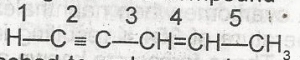
The acidic hydrogen in the compound is the hydrogen attached to carbon number
- A. 5
- B. 4
- C. 3
- D. 1
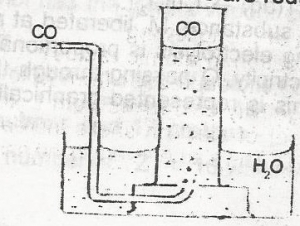
Carbon (ll) oxide may be collected as shown above because
- A. is heavier than air
- B. is less dense than air
- C. is insoluble in water
- D. burns in oxygen to form carbon (IV) oxide
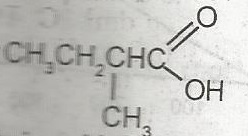
The IUPAC name for
- A. 2-methylbutanoic acid
- B. 2-methyl-1- hydroxyketone
- C. 2-methyl-1-hydroxy aldehyde
- D. 2-methylpentanoic acid
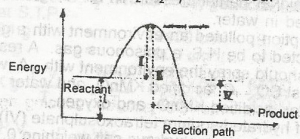
The diagram above shows the reaction path of an exothermic reaction. The heat of reaction is represented by
- A. l
- B. ll
- C. lll
- D. lV
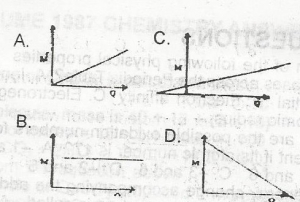
Use the graph above to answer this question. The mass of a substance, M, liberated at an electrode during electrolysis is proportional to the quantity of electricity, Q passing through the electrolyte. This is represented graphically by.
- A. A
- B. B
- C. C
- D. D
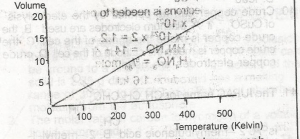
Which of the gas laws does the above graph illustrate?
- A. Boyle
- B. Charles
- C. Graham
- D. Gay-Lussac
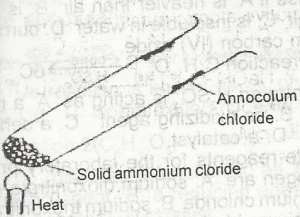
In the experiment above, ammonium chloride crystals deposit on the walls of the tube is as a result of
- A. evaporation
- B. recrystallization
- C. sublimation
- D. fractional precipitation
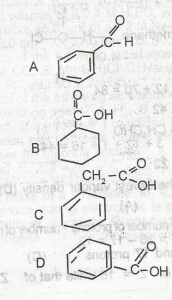
Use the option above to answer this question. The structure of benzoic acid is
- A. A
- B. B
- C. C
- D. D

The times taken for iodine to be liberated in the reaction between sodium thiosulphate and hydrochloric acid at various temperature are as follows. These results suggest that
- A. for a 10o rise in temperature, rate of reaction is double
- B. for a 10 o rise in temperature, rate of reaction is halved
- C. time taken for iodine to appear does not depend on temperature
- D. for a 10 o rise in temperature rate of reaction is tripled

Use the option above to answer this question. Which is the correct set of results for tests conducted respectively on fresh lime juice and ethanol.
- A. A
- B. B
- C. C
- D. D
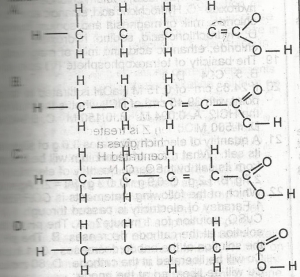
Use the options above to answer this question. Which is the structural formula for pent-2-enoic acid?
- A. A
- B. B
- C. C
- D. D

The tabulated results below were obtained by titration 10.0 cm3 of water with soup. The titration was repeated with the same sample of water after boiling. The ratio of permanent to temporary hardness is
- A. 1.5
- B. 1.4
- C. 4.1
- D. 5.1
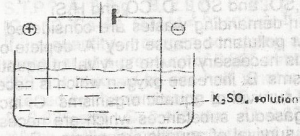
In the electrolysis of aqueous solution of K2SO 4 in the above cell, which species migrate to the anode?
- A. SO2-OH -
- B. K + and SO 4 2-
- C. OH and HO
- D. H3O + and K
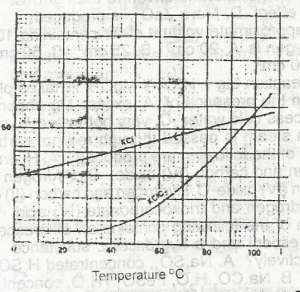
In the solubility curve above, water at 98oC is saturated with KCL and KCIO 3 What is the percentage of KCL impurity in the crystals formed when the solution is cooled to 30 oC?
- A. 51.5
- B. 45.5
- C. 34.5
- D. 26.5

Use the following option above to answer this question. Which of the following is the functional group of carboxylic acids?
- A. A
- B. B
- C. C
- D. D
- E. E
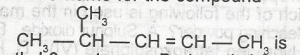
The I.U.P.A.C name for the compound
- A. 2-methyl-3-pentene
- B. 4-methyl-2-pentane
- C. 2-methyl-2-pentene
- D. 4-methylpent-2-ene
- E. 2-methyl-3-pentane
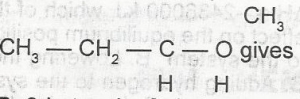
The oxidation of
- A. 2-butanone
- B. 2-butanal
- C. butane
- D. butanoic acid
- E. 3-butanal
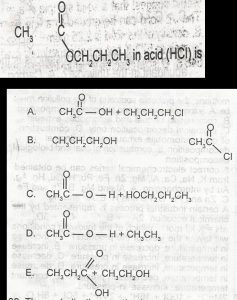
Use the above options to the question. The products formed on hydrolysis of
- A. A
- B. B
- C. C
- D. D
- E. E
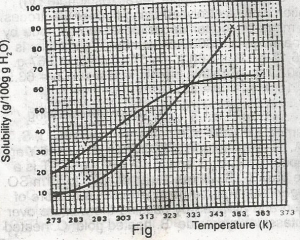
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
- A. 0.2 moles
- B. 0.7 moles
- C. 1.5 moles
- D. 2.0 moles
- E. 3.0 moles

The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If 80 g each of X and Y are taken up in 100 g of water at 353 K we shall have
- A. only 10g of X undissolved
- B. only 16 g of Y undissolved
- C. 10 g of X and 16 g of Y undissolved
- D. all X and Y dissolved
- E. all X and Y undissolved
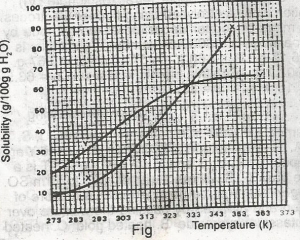
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. At room temperature (300 K)
- A. Y is twice as soluble as X
- B. X is twice as soluble as Y
- C. X and Y are soluble to the same extent
- D. X is three times as soluble as Y
- E. Y is three times as soluble as X


