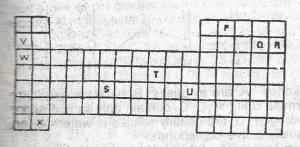
Figure 1 above shows part of the periodic Table which of the elements belongs to the p-block?
- A. S, T and U
- B. V, W and X
- C. S and T only
- D. P, Q and R
- E. V, W, X and S
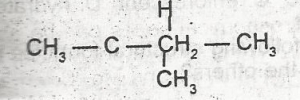
The I.U.P.A.C name for the compound is
- A. isopropylethene
- B. acetylene
- C. 3- methylbutane
- D. 2-methylbutane
- E. 5-methylpentane
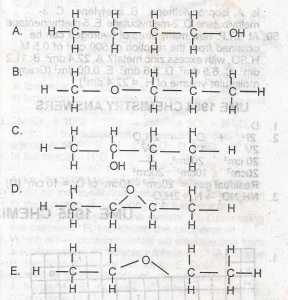
Use the diagram above to answer this question
Which of the following structural formula is NOT isomeric with the others?
- A. A
- B. B
- C. C
- D. D
- E. E
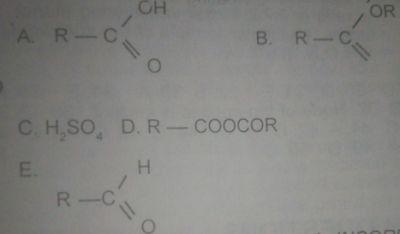
USE THE DIAGRAM ABOVE TO ANSWER THIS QUESTION.
Which of the following represents a carboxylic acid?
- A. A
- B. B
- C. C
- D. D
- E. E
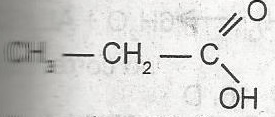
The name of the compound above is
- A. acetic acid
- B. propanal
- C. propanol
- D. ethanoic acid
- E. propanoic acid
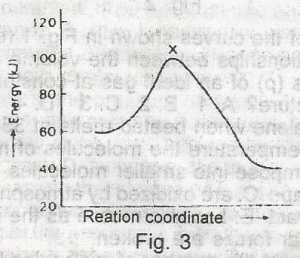
The diagram above (Fig.3) shows the energy profile for the reaction A + B + = C + D From this diagram, it is clear that the reaction is
- A. spontaneous
- B. isothermal
- C. adiabatic
- D. exothermic
- E. endothermic
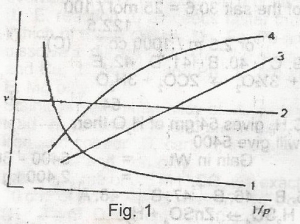
Which of the curves shown in the figure given represents the relationships between the volume(V) and pressure(P) of an ideal gas at constant temperature
- A. 1
- B. 2
- C. 3
- D. 4
- E. 1 and 3
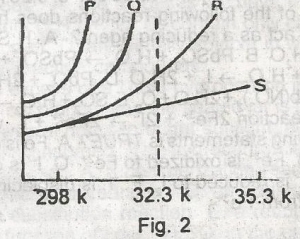
The solubility curves of four substances are shown in the figure above. Which of the four substances would crystallize from a saturated solution cooled from 353K(80oC)to 323K(50C)?
- A. P and Q
- B. P and R
- C. P and S
- D. R and S
- E. Q and R
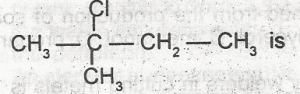
The i.u.p.A.C name for the compound
- A. 2-chloro-isopentane
- B. 3-chloro-isopentane
- C. 2-chloro-2-methylbutane
- D. 1-chloro-2,2- dimethylpropane
- E. 2-chloro-2-ethylpropane
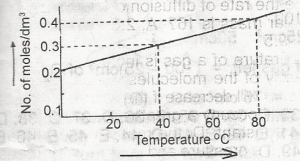
The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be
- A. 10 g
- B. 12 g
- C. 14 g
- D. 16 g
- E. 18 g
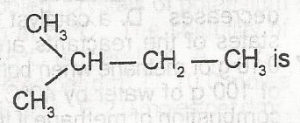
The IUPAC name for
- A. 1-methlpentane
- B. 3-methylbutane
- C. 2-methylbutane
- D. 1-dimethylpropane
- E. 2-methylpentane
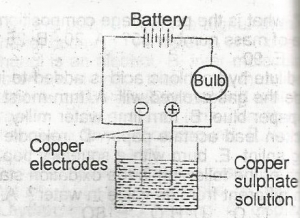
Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed?
- A. The bulb lights and copper is deposited at both electrodes
- B. the bulb lights and copper is deposited at the anode and disappears from the cathode
- C. the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode
- D. the bulb lights and copper is deposited at the cathode and disappears from the anode
- E. the bulb lights but no additional changes are observed
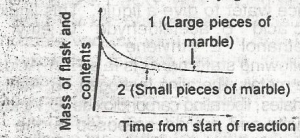
The following graph demonstrates the rate of reaction between calcium carbonate (marble)and dilute hydrochloric acid. The graph shows that the rate of reaction is initially greatest when
- A. the initial slope of the curve is steepest and this occur when small pieces of marble are used and is due to a small surface area of reactant
- B. the occur when large pieces of marble are used and is due to a small surface area of reactant
- C. the initial slope of the curve is steepest and this occurs when small pieces of marble are used and is due to a large surface area of reactant
- D. the initial slope of the curve is steepest and this occur when large surface area of reactant
- E. the initial slope of the curve is least steep and this occur when small pieces of marble are used and is due to the increased surface area of the reactant

Which of the following is the correct order in the above diagram increasing boiling point, of the isomeric \(C_{5}H_{12}\) compounds?
- A. A
- B. B
- C. C
- D. D
- E. E
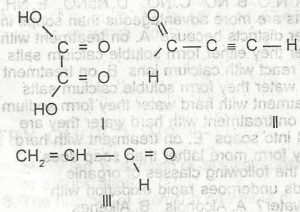
Which of the following compounds will take up the molecules of bromine?
- A. l
- B. ll
- C. lll
- D. l and ll
- E. l and lll
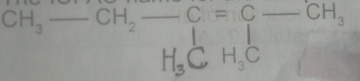
The IUPAC name for the compound is
- A. 2,3-dimethylpent-2,3-ene
- B. 2, 3- dimethylpent-2-ene
- C. 3, 4-dimethylpent-3-ene
- D. 3,4-dimethylpentene
- E. 3,4-dimethlhept-2-ene
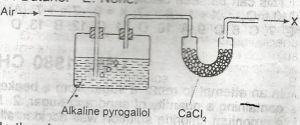
In the above experiment X is?
- A. pure nitrogen
- B. a mixture of nitrogen and oxygen
- C. a mixture of nitrogen and carbondioxide
- D. a mixture of oxygen and inert gases
- E. a mixture of nitrogen and inert gases
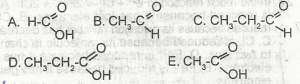
Which of the structural formula above is ethanoic acid is
- A. A
- B. B
- C. C
- D. D
- E. E
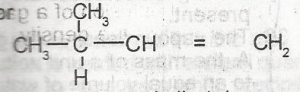
The IUPAC name for
- A. 2-methylbut-3-ene
- B. 2-methylbut-4-ene
- C. 3-methylbut-2-ene
- D. 3-methylbut-1-ene
- E. 3-methylpent-1-ene
30 cm3 of 0.1 M Al (NO3)3 SOLUTION IS RECTED WITH 100cm3 of 0.15M of NaOH solution. Which reactant is in excess, and by now much ?
- A. NaOH solution by 70 cm3
- B. NaOH solution, by 60 cm3
- C. NaOH solution by 40 cm3
- D. Al(NO3)3 solution by 20 cm3
- E. Al(NO3)3 solution by 10 cm3
\(Zn + H_2 SO_4 → ZnSO_4 + H_2\)
in the above reaction, how much zinc will be left undissolved if 2.00g of zinc is treated with 10cm^3 of 1.0 M of \(H_2SO_4\)?
[Zn =65, s = 32, O = 16, H = 1]
- A. 1.35g
- B. 1.00g
- C. 0.70g
- D. 0.65g
- E. 0.00g


