In the reactions
(I) H2 (g) + 1/2O2(g) → H2O(l); ∆H = -286 KJ
(II) C(s) + O2g) → CO2 (g); ∆H = -406 KJ the equations imply that
- A. more heat is absorbed in (l)
- B. more heat is absorbed in (ll)
- C. less heat is evolved in (l)
- D. reaction (ll) proceeds faster than (l)
- E. reaction (l) proceeds faster than (ll)
Solutions X, Y, and Z have PH values 3.0. 5. 0 and 9. 0 respectively. Which of the following statements is correct?
- A. All the solutions are acidic
- B. All the solutions are basic
- C. Y and Z are more acidic than water
- D. Y is more acidic than X
- E. Z is the least acidic
An element is electronegative if
- A. it has a tendency to exist in the gaseous from
- B. its ions dissolve readily in water
- C. it has a tendency to lose electrons
- D. it has a tendency to gain electrons
- E. it readily froms covalent bonds
Crude petroleum pollutant usually seen on some Nigerian creeks and waterways can be dispersed or removed by
- A. heating the affected parts in order to boil off the petroleum
- B. mechanically stirring to dissolve the petroleum in water
- C. pouring organic solvents to dissolve the petroleum
- D. spraying the water with stergents
- E. cooling to freeze out the petroleum
Which of the following happens during the electrolysis of molten sodium chloride?
- A. Sodium ion loses an electron
- B. Chlorine atom gains an electron
- C. Chlorine ion gains an electron
- D. Sodium ion is oxidized
- E. Chloride ion is oxidized
Sodium sulphate decahydrate (Na2SO4.10 H2O) on exposure to air loses all its water of crystallization.
The process of loss is known as
- A. efflorescence
- B. hygroscopy
- C. deliquescence
- D. effervescence
- E. dehydration
A piece of burning sulphur will continue to burn in a gas jar of oxygen to give misty fumes which readily dissolved in water. The resulting liquid is
- A. sulphur (VI) trioxide
- B. tetraoxosulphate (VI) acid
- C. trioxosulphate (IV) acid
- D. dioxosulphate (II) acid
- E. hydrogen sulphide
How many isomeric forms are there for the molecular formula C3H6Br2?
- A. 1
- B. 2
- C. 3
- D. 4
- E. 5
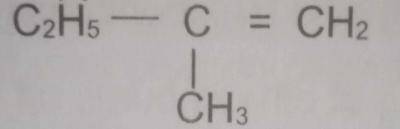
The correct name of the compound with the above structural formula is
- A. 2-methylbut -1-ene
- B. 2-methylbut -2-ene
- C. 2-methylbutane
- D. 2-ethylprop-1 -ene
- E. 2-ethylprop-2-ene
In the reaction Fe + Cu2+ \(\to\) Fe2+ + Cu,iron displaces copper ions to from copper. This is due to the fact that
- A. irn is in the metallic from while the copper is in the ionic from
- B. the atomic weight of copper is greater than that of iron
- C. copper is greater than iron
- D. copper metal has more electrons than iron metal
- E. iron is higher in the electrochemical series than copper
In which of the following processes is iron being oxidized?
1 Fe + H2SO4 → FeSO4 +H2
2 FeSO4 + H2S → FeS + H2SO4
3 2FeCI2 + Cl2 → 2FeCl3
4 2FeCl3 + SnCl2 + → 2FeCl2 + SnCl4
- A. 1 only
- B. 2 only
- C. 3 only
- D. 1 and 3
- E. 2 and 4
In the reaction M + N P;∆H = + QKJ. Which of the following would increase the concentration of the product?
- A. Decreaseing the concentration of N
- B. Increasing the concentration of P
- C. Adding a suitable catalyst
- D. Decreasing the temperature
- E. Increasing the temperature
The colour imparted to a flame by calcuium ion is
- A. green
- B. blue
- C. brick-red
- D. yellow
- E. lilac
Which of the following relationships between the pressure P, the volume V and the temperature T, represents an ideal gas behaviour?
- A. P \(\alpha\) VT
- B. P \(\alpha\) T/V
- C. PT \(\alpha\) V
- D. PV \(\alpha\) I/T
- E. P \(\alpha\) V/T
A mixture of common salt, ammonium chloride and barium sulphate can best be separated by
- A. addition of water followed by filtration then sublimation
- B. addition of water followed by sublimation then filtration
- C. sublimation followed by addition of water then filtration
- D. fractional distillation
- E. fractional crystallization
An unknown organic compound X has a relative molecular mass of 180. It is a colourless crystaline solid, readily soluble in water. X contains the elements C, H and O in the atomic ratio 1: 2:1. The compound has a sweet teste and melts on heating. In the presence of yeast and in the absence of air, X is converted to compound Y and a colourless gas. Compound Y reacts with sodium metal to produce a gas Z which gives a ‘pop’ sound with a glowing splint. Y also reacts with ethanoic acid to give a sweet smelling compound W.
Reaction of X with yeast forms the basis of the
- A. plastic industry
- B. textile industry
- C. brewing industry
- D. soap industry
- E. dyeing industry
An unknown organic compound X has a relative molecular mass of 180. It is a colourless crystaline solid, readily soluble in water. X contains the elements C, H and O in the atomic ratio 1: 2:1. The compound has a sweet teste and melts on heating. In the presence of yeast and in the absence of air, X is converted to compound Y and a colourless gas. Compound Y reacts with sodium metal to produce a gas Z which gives a ‘pop’ sound with a glowing splint. Y also reacts with ethanoic acid to give a sweet smelling compound W.
The molecular formula of X is
- A. C12H22O11
- B. C6H12O6
- C. C3H6O 3
- D. C7H14O7
- E. C12H6O4
An unknown organic compound X has a relative molecular mass of 180. It is a colourless crystaline solid, readily soluble in water. X contains the elements C, H and O in the atomic ratio 1: 2:1. The compound has a sweet teste and melts on heating. In the presence of yeast and in the absence of air, X is converted to compound Y and a colourless gas. Compound Y reacts with sodium metal to produce a gas Z which gives a ‘pop’ sound with a glowing splint. Y also reacts with ethanoic acid to give a sweet smelling compound W.
Compound W is
- A. a soap
- B. an oil
- C. an alkane
- D. an ester
- E. sucrose
The three-dimensional shape of methane is
- A. hexagonal
- B. trigonal
- C. linear
- D. tetrahedral
- E. cubical
Some copper (ll) sulphate pentahydrate (CuSO4 5H2O), was heated at 120 oC with the following results. Wt of crucible = 10.00 g; Wt of crucible + CuSO4 5H2O =14.98g; Wt of crucible + residue = 13.54 g. How many molecules of water of crystalization were lost?
[H = 1, Cu = 63.5, O = 16, S = 32]
- A. 1
- B. 2
- C. 3
- D. 4
- E. 5
If an element has the electronic configuration 1s 2 2s2 2p3 3s2 3p4, it is
- A. a metal
- B. an alkaline earth metal
- C. an s-block element
- D. a p-Block element
- E. a transition element


