Which of the following are the products of the reaction between CH3COOH and CI2 in sunlight?
- A. CICH2COOH + HCI
- B. CH3COCI + HOCI
- C. CH3COOCI + HCI
- D. CH3COCI + HO2
Which of the following statements is TRUE of the complete hydrolysis of a glycerine by sodium hygroxide?
- A. 3 moles ofNaOH are required for each mole of glyceride
- B. 3 moles of glycerol are produced
- C. Only one mole of soap is formed
- D. concentrated H2SO4 is essential for the completion of the reaction
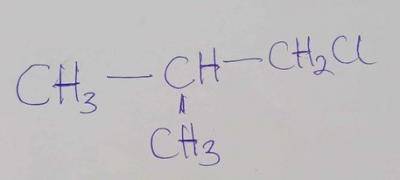
Is known as?
- A. 1-chloro-2-methylbutane
- B. 1-chloro-2-methylpropane
- C. 2-chloromethylpropane
- D. 1-chloro-2, 2-dimethylethane
A certain liquid has a high boiling point. It is viscous, non-toxic, miscible with water being very hygroscopic. This liquid is most likely to be?
- A. CH3CH2CH2HO
- B. CH3CHOHCH3
- C. CH3CH2CHOHCH3
- D. CH2OHCHOHCH2OH
One advantages of detergents over soap is that detergents?
- A. are easier to maufacture
- B. foam more than soap
- C. form soluble salts with hard water
- D. are able to deter germs more than soap
Ethene when passed into concentrated H2SO4 is rapidly absorbed. The product is diluted with water and then warmed to produce?
- A. ethanol
- B. diethyl ether
- C. ethanal
- D. diethyl sulphate
How many grams of bromine will be required to completely react with 10 g of propyne?
(C = 12, H = 1, Br = 80)
- A. 20 g
- B. 40 g
- C. 60 g
- D. 80 g
Which of the following compounds can exist as geometric isomers?
- A. 2-methylbut-2ene
- B. But-2-ene
- C. But-1-ene
- D. CI-C-Br
Copper (II) tetraoxosulphate (IV) is widely used as a?
- A. fertilizer
- B. fungicide
- C. disinfectant
- D. purifier
Which of the following metals can be prepared in samples by the thermal decomposition of their trioxonitrate (V) salts?
- A. Copper and mercury
- B. Silver and copper
- C. Mercury and silver
- D. Magnesium and mercury
Which of the following, in clear solution, forms a white precipitate when carbon (IV) oxide is bubbled into it for a short time?
- A. KOH
- B. NaOH
- C. Ca(OH)2
- D. AI(OH)3
Addition of aqueous ammonia to a solution of Zn++ gives a white precipitate which dissolves in an excess of ammonia because?
- A. zinc is amphoteric
- B. zinc hydroxide is readily soluble
- C. zinc forms a complex which is readily soluble in excess ammonia
- D. ammonia solution is a strong base
Oxygen gas can be prepared by heating?
- A. ammonium trioxonitrate (V)
- B. ammonium trioxonitrate (III)
- C. ppotassium trioxonitrate (V)
- D. mamgamese (IV) oxide
Which of the following salts can be melted without decomposition?
- A. Na2CO3
- B. CaCO3
- C. MgCO3
- D. ZnCO3
Which of the following gases will rekindle a brightly glowing splint?
- A. NO2
- B. NO
- C. N2O
- D. CI2
Which of the following equilibria is unaffected by a pressure change?
- A. 2NaCI(s) ↔ 2Na (I) + CI2(g)
- B. H 2(g) + I2(g) ↔ 2HI(g)
- C. 2O3(g) ↔ 3O2(g)
- D. 2NO2(g) ↔ N2O4(g)
NO(g) + CO(g) ↔ (1)/(2)N2(g) + CO2(g) ∆H = 89.3 kj.
What conditions would favour maximum conversion of nitrogen (II) oxide and carbon (II) oxide in the reaction above?
- A. Low temperature and high pressure
- B. High temperature and low pressure
- C. High temperature and high pressure
- D. Low temperature and low pressure
1.1 g of CaCI2 dissolved in 50 cm3 of water caused a rise in temperature of 3.4°C. The heat of reaction, ∆H for CaCI2 in kj per mole is?
(Ca = 40, CI = 35.5, specific heat of water is 4.18 jk-1)
- A. -71.1
- B. -4.18
- C. +71.1
- D. +111.0
Magnesium (IV) oxide is known to hasten the decomposition of hydrogen peroxide. Its main action is to?
- A. increase the surface area of the reactants
- B. increase the concntration of the reactants
- C. lower the activation energy for the reaction
- D. lower the heat of reaction, ∆H, for the reaction
2H2S(g) + SO2(g) → 3S(s) + 2H2O(I). The above reaction is?
- A. a redox reaction H2S is the oxidant and SO2 is the reductant
- B. a redox reaction in which SO2 is the oxidant and H2S is the reductant
- C. not a redox reaction because there is no oxidant in reaction equation
- D. not a redox because there is no reductant in the reaction equation
MnO–4 + 8H+ + ne → Mn++ + 4H2O. Which is the value of n in the reaction above?
- A. 2
- B. 3
- C. 4
- D. 5


