On the basis of the electrochemical series, which of these ions will show the greater tendency to be discharged at the cathode in an electrolytic cell
- A. cu\(^{2+}\)
- B. sn\(^{2+}\)
- C. fe\(^{2+}\)
- D. zn\(^{2+}\)
A chemical widely used as a fertilizer is?
- A. galena
- B. bauxite
- C. emerald
- D. nitrochalk
Addition of sodium chloride to water to form a solution would lead to?
- A. increase in freezing point and increase the boiling point
- B. increase in freezing point and decrease the boiling point
- C. decrease in freezing point and decrease the boiling point
- D. decrease in freezing point and increase the boiling point
An organic compound which liberate carbon(iv)oxide from trioxocarbonate(iv) solution is likely to be?
- A. C\(_2\)H\(_5\)OH
- B. C\(_3\)H\(_4\)
- C. C\(_6\)H\(_6\)
- D. CH\(_3\)COOH
The sulphide that is commonly used in coating electric fluorescent tubes is?
- A. iron(ii)Sulphide
- B. tin(ii)sulphide
- C. Zinc Sulphide
- D. lead(iv) Sulphide
How many neutrons are present in atom with mass number and atomic number 37 and 17 respectively?
- A. 18
- B. 20
- C. 37
- D. 17
The following non-metal form acidic oxides with oxygen except?
- A. phosphorus
- B. sulphur
- C. carbon
- D. chlorine
In the preparation of salts, the method employed will depend on the?
- A. composition
- B. dissociating ability
- C. stability to heat
- D. precipitating ability
SO\(_2\) + O\(_2\) → 2SO\(_3\)
In the reaction above, the most suitable catalyst is?
- A. chromium(vi)oxide
- B. iron(iii)oxide
- C. copper(i)oxide
- D. vanadium(v)oxide
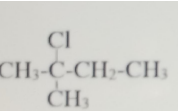
The IUPAC nomenclature of the structure is?
- A. 3-chloro-3-methylbutane
- B. 2,2,-dichloro-3-methylbutane
- C. 3-methylchlorobutane
- D. 2-chloro-2-methylbutane
The sub-atomic particles located in the nucleus of an atom are?
- A. neutron and proton
- B. proton and electron
- C. proton and ions
- D. neutron and electron
(a) (i) Define the term Avogadro’s number.
(ii) If 2.30 g of an oxide of nitrogen, x, contains 3.01 x 10\(^{22}\) molecules, calculate the molar mass of x.
(iii) Deduce the formula of x. N, =6.02 x 10″, N =14.0, O = 16.0]
(b)(i) Describe briefly what happens when each of the following substances are added to water:
(I) CCI\(_{4}\); (II) SiCI\(_{4}\),
(ii) Explain briefly why the reactions in (a)(i), (b)(i), (I) and (b)(ii) (II) are different Study the diagram below and answer the questions that follow.
(c) Study the diagram below and answer the questions that follow.

(i) What is the set up used for?
(a)(i) Define the first ionization energy of an element
(ii) Consider the following table and use it to answer te question that follows
| Element | Li | Be | b | C | N | O | F | Ne |
| Atomic number | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1st I.E/kj mol\(^{-1}\) | 520 | 900 | 801 | 1086 | 1402 | 1314 | 1681 | 2081 |
Explain briefly why the first ionization energy of B is less than that of Be despite the fact that the atomic number of B is greater than that of Be.
(b) When Titanium chloride was electrolysed by passing 0.12 A current through the solution for 500 seconds, 0.015 g of titanium was deposited. What is the charge on the titanium ion?
[ IF= 96500 C, Ti= 48.0 ]
(c)(i) Aluminium can be obtained by the application of electrolysis. State the electrolyte which yields aluminium on electrolysis.
(ii) Name two major factors which would favour the siting of an aluminium smelter in a country.
(d)(i) Define the term paramagnetism.
(ii) Consider the following ions: 24\(^{Cr ^{2+}}\), 24\(^{Cr^{6+}}\)
(I) Deduce the number of unpaired electrons in each of the ions.
(II) State which of the ions will have a greater power of paramagnetism
(l) Give a reason for the answer stated in (d)(ii)(II)
(a)i. With the aid of an equation, explain briefly why aluminum metal is not affected by air.
(ii) In the extraction of aluminum from bauxite, state the:
– substance used for purifying the ore;
– composition of the mixture electrolyzed.
(b) ZnO is an amphoteric oxide. Write equations to illustrate this statement.
(c)i) List three uses of sodium trioxocarbonate(IV).
(ii) Explain briefly why a solution of trioxonitrate(V) acid turns yellowish on storage for some time.
(ii) Describe briefly how trioxonitrate(V) ions could be tested for in the laboratory.
(d) Write balanced chemical equations for the preparation of hydrogen chloride.
(i) using concentrated H\(_{2}\)SO\(_{4}\):
(ii) by direct combination of its constituent elements.
(iii) State one use of hydrogen chloride.
(a) What is the structure of:
(i) graphite:
(ii) diamond
(ii) Explain briefly why diamond is hard and a non-conductor of electricity while graphite is soft and an electrical conductor,
(b)i. State what is achieved at each of the following stages in the purification of town water supply:
– aeration;
– screening
– sedimentation.
(ii) Name two substances responsible for hardness in water.
(iii) State two methods for the removal of hardness in water.
(iv) Give one disadvantage of hard water
(c)i). Describe briefly the extraction of tin from its ore.
(ii) Write a balanced chemical equation for the reaction.
(iii) Write an equation for the reaction of tin with:
– oxygen;
– chlorine.
(a) In an experiment, 25.0 cm\(^{3}\) of H\(_{2}\)SO\(_{4}\) completely neutralized 24.0 cm\(^{3}\) of a 0.1 50 mol dm\(^{-3}\) aqueous KOH using a suitable indicator.
(i) Write a balanced chemical equation for the reaction.
(ii) Calculate the concentration of the acid solution.
(b)i. A burning magnesium ribbon was placed in a gas jar containing Carbon (IV) oxide.
– Write an equation for the reaction.
– Explain briefly why the magnesium ribbon burns in carbon(lV) oxide although the gas does not support combustion.
– Calculate the percentage mass of nitrogen in magnesium trioxonitrate (V). |N = 140. O = 16.0. Mg = 24.01]
(c) Consider the following organic compound: CH\(_{3}\)CH\(_{2}\)CH = CHCOOH.
(i) State two chemical reactions which could he sed te identify the compound.
(ii) What would be observed in each of the reactions stated in c(i)
(d) Describe briefly how soap is manufactured using pellets of sodium hydroxide and vegetable oil
(e) Define the term electronegativity
(a)i. State two characteristics of a homologous series.
(ii) Explain briefly why there are differences in the reaction of ethane and ethene.
(b) When crystals of sodium chloride were warmed with concentrated tetraoxosulphate( VI) acid, a gas was evolved.
(i) Name the gas.
(ii) State two physical properties of the gas.
(iii) Write a balanced chemical equation for the reaction.
(c)i. What are hydrocarbons?
(ii) State two natural sources of hydrocarbons.
(iii) A hydrocarbon contains 83% of carbon by mass. Calculate its empirical formula. [H=1.0, C=12.0]
(d) Draw and label a diagram of a set-up that could be used to electroplate a copper ornament with silver
(a) Distinguish between molecular formula and structural formula
(b) List three factors that determine the ionization energy of an atom.
(c) State the two conditions necessary for the establishment of a chemical equilibrium
(d) Consider the following table
| Element | A | B | C |
| Ionization energy KJ mol\(^{-1}\) | 619 | 518 | 594 |
(d)(i) State which of the elements is the strongest reducing agent.
(ii) Give a reason for the answer stated in (d)(i)
(e) State Graham’s law of diffusion
(f) Consider the following salts: Mg(NO\(_{3}\))\(_{2}\), CaCO\(_{3}\), Na\(_{2}\)SO\(_{4}\). State which of the salts is/are:
(i) readily soluble in water:
(ii) insoluble in water.
(g) Classily each of the following products as addition polymer or condensation polymer:
(i) protein:
(ii) perspex:
(iii) nylon.
(h) Define atomic radius.
(i) Explain briefly why ethanol has a higher boiling point than propane even though they both have comparable molar masses.
(j) State three significance of the pH value in everyday life.
Which of the following scientists discovered the electrons?
- A. Joseph J. Thompson
- B. James Chadwick
- C. Amedeo Avogadro
- D. Ernest Rutherford
A coordinate covalent bond could be formed between?
- A. NH\(_{3}\) and PCl\(_{3}\)
- B. BCl\(_{3}\) and AlCl\(_{3}\)
- C. BCl\(_{3}\) and NH\(_{3}\)
- D. H\(^{+}\) and AlCl\(_{3}\)
Electrovalent compounds normally ————
- A. have low boiling points
- B. have mobile electrons
- C. conduct electricity in the solid state
- D. dissolve in polar solvent


