In a reaction: \(SnO_{2} + 2C → Sn + 2CO\) the mass of coke containing 80% carbon required to reduce 0.302 kg of pure tin oxide is?
(Sn – 119, O – 16, C – 12).
- A. 0.40 kg
- B. 0.20 kg
- C. 0.06 kg
- D. 0.04 kg
Which of the following is a physical change?
- A. The bubbling of chlorine into water
- B. The bubbling of chlorine into a jar contanining hydrogen
- C. The dissolution of sodium chloride in water
- D. The passing of steam over heated iron
Which of the following statements is true?
- A. Metals conduct electricity while non-metals do not
- B. Metals have high density, non-metals have low density
- C. Metals form basic oxides, non-metals form acidic oxides
- D. In the electro chemical series, metals are above hydrogen while non-metals are below hydrogen
- E. Metals lose elections while non-metals gain electron during chemical reactions
Which of the following statements is an exception in the assumptions of the kinetic theory of gases?
- A. Gases are composed of many elastic particles
- B. The particles are of negligible size
- C. The particles are in constant random motion
- D. The particles are of negligible mass
- E. The particles collide with each other
How many grams of hydrogen gas will be liberated when 6g of magnesium ribbon dissolves in 500cm3 of 6M HCl?
(Mg=24, H=1, Cl=35.5)
- A. 1.2 g
- B. 0.7 g
- C. 0.5 g
- D. 0.3 g
- E. 0.12 g
Under high pressure, real gases do not obey gas laws because their molecules?
- A. have become more energetic
- B. have become less energetic
- C. have become smaller in size
- D. decompose into atoms
- E. start repelling each other
Which of the following reactions is correct as as written?
- A. FeCl2 + 3NH4OH → Fe(OH)3 + 3NH4Cl
- B. Pb2O3 + 4HCl → PbCl2 + 2H2O
- C. PbNO3 + H2S → PbS + 2HNO3
- D. NaOH + H2SO4 → NaHSO4 +H2O
- E. 2NH4NO3 → N2 + 2H2O
If 1 litre of 2.2M sulphuric acid is poured into a bucket containing 10 litre of water,and resulting solution mixed thoroughly, the resulting sulpuric acid concentration will be?
- A. 2.2 M
- B. 1.1 M
- C. O.22 M
- D. 0. 11 M
- E. 0.20 M
On heating, under suitable conditions, 1 litres of monoatomic gas,X, combines with 11/2 litres of an oxide, What is the formula of the oxide.
- A. XO3
- B. X2O3
- C. X3O2
- D. XO2
- E. None of the above
A sample of an oxide of mercury weighed 1.00g. When this was heated, oxygen was given off, and 1.00g of mercury metal only was left. What is the formula of the oxide?
- A. Hg2O
- B. HgO2
- C. HgO2
- D. HgO
- E. None of the above
10cm3 of CO is mixed and sparked with 100cm3 of air containing 21% O2. If all the volumes measured at S.T.P. the volume of resulting gases would be?
- A. 90cm3
- B. 100cm3
- C. 105cm3
- D. 110cm3
- E. 115cm3
A solution X on mixing with AgNO3 solution, gives a white precipitate solution in NH3. A Solution Y, when added to X, also gives a white precipiate which is soluble on boiling. Solution Y contains?
- A. Ag+ion
- B. Pb2+ion
- C. Pb4+ion
- D. Zn2+ion
- E. Al3+ion
The alloy duralumin is used for the building of aeroplanes because it?
- A. asborbs heat readily from sure thereby preventing the inside of the plane from getting cold at high attitudes
- B. reflects sunlight readily, thereby keeping the inside of the plane cool
- C. is extremely durable and possesses reflective ability
- D. is easily the lightest building material
- E. possesses both strenght and lightness
In an attempt to remove sugar from a breaker containing a quantity of sand and sugar, 2 M ammonium chloride solution was accidentally added instead of water. Which of the following method could be used to remove the ammonium chloride from the mixture?
- A. Fractional distillation
- B. crystallization
- C. Filtration followed by evaporation
- D. Evaporation followed by sublimation
- E. Filtration followed by sublimation
The size (diameter) of five elements are in the order R < T < W < X < Y, Y being the largest. If each atom has an electron situated on its circumference, and neglecting other factors, which of the atoms will lose its electron most reluctantly?
- A. R
- B. T
- C. W
- D. X
- E. Y
Five compounds R,T,W,X and Y form the following compounds: a basic hydride R.H, an acidic hydride YH, atmospheric oxide W2O3, XO2. which of the elements is an alkali
- A. R
- B. T
- C. W
- D. X
- E. Y
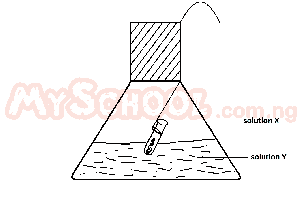
An experiment test of the Law of conservation of Mass is illustrated in the diagram. In practice, the flask is weighed before and after reaction between solutions X and Y. Which of the following pairs of solutions will be unsuitable for the experiments
- A. X = hydrochloric acid; Y = silver nitrate
- B. X = barium chloride; Y = dilute sulphuric acid
- C. X = hydrochloric acid; Y = sodium hydroxide
- D. X = hydrochloric acid; Y = lead nitrate
- E. X = hydrochloric acid; Y = sodium carbonate
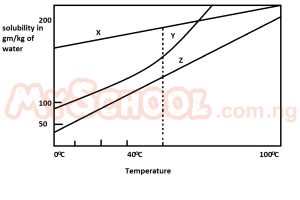
Consider the solubility curves of three salts X, Y and Z given in the diagram. If each solution of the salt contains 200 g, and is heated to 100oC, which solution or solutions will deposit 100 g of the solute when suddenly cooled to 0oC?
- A. X only
- B. Y only
- C. Z only
- D. X and Z
- E. Y and Z
A solid X when heated gives off a brown gas. If X is soluble in excess sodium hydroxide solution but insoluble in excess ammonium hydroxide solution, then X is
- A. basic lead carbonate
- B. lead (II) nitrate
- C. sodium carbonate
- D. zinc nitrate
- E. sodium nitrate
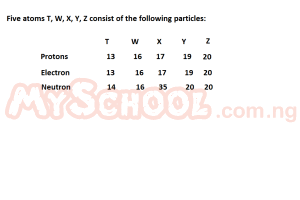
Which of the five atoms can be described by the following properties: Relative atomic mass is greater than 30 but less than 40, it has odd atomic number and forms a unipositive ion in solution?
- A. T
- B. W
- C. X
- D. Y
- E. Z
with conc H2SO4 as catalyst
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
- A. esterification
- B. Condensation
- C. saponification
- D. neutralization


