The function of zinc electrode in a galvanic cells is that it
- A. undergoes reduction
- B. serves as the positive electrode
- C. production of electons
- D. uses up electrons
A chemical reaction which the hydration energy is greater than the lattice energy is referred to as
- A. a spontaneous reaction
- B. an endothermic reaction
- C. an exothermic reaction
- D. a reversible reaction
Which of the following is a deliquescent compound?
- A. NaNO3
- B. CaCl2
- C. CuO
- D. Na2CO3.10H2O
An effect of thermal pollution on water bodies is that the
- A. volume of water reduces
- B. volume of chemical waste increase
- C. level of oxides of nitrogen increase
- D. level of oxygen reduces
Which of the following is NOT an alkali?
- A. NH3
- B. Mg(OH)2
- C. Ca(OH)2
- D. NaOH
How many cations will be produced from a solution of potassium aluminium tetraoxosulphate (VI)?
- A. 3
- B. 4
- C. 1
- D. 2
CH3COOH(aq) + OH–(aq) ↔ CH3COO–(aq) + H2O(l)
In the reaction above ,CH3COO–(aq) is
- A. conjugate base
- B. acid
- C. base
- D. conjugate acid
N2O4(g) ↔ 2NO2(g) ΔH = +ve
In the reaction above, an increase in temperature will
- A. increase the value of the equilibrium constant
- B. decreases the value of the equilibrium constant
- C. increase in the reactant production
- D. shift the equilibrium to the left
Which of the following noble gases is commonly found in the atmosphere?
- A. Xenon
- B. Neon
- C. Helium
- D. Argon
A suitable solvent for iodine and naphthalene is
- A. carbon (IV) sulphide
- B. ethanol
- C. water
- D. benzene
Hardness of water is mainly due to the presence of
- A. calcium hydroxide or magnesium hydroxide
- B. calcium trioxocarbonate (IV) or calcium tetraoxosulphate (VI)
- C. sodium hydroxide or magnesium hydroxide
- D. calcium chloride or sodium chloride salts
What type of bond exits between an element X with atomic number 12 and Y with atomic number 17?
- A. Electrovalent
- B. Metallic
- C. Covalent
- D. Dative
The importance of sodium aluminate (III) in the treatment of water is to
- A. cause coagulation
- B. neutralize acidity
- C. prevent goitre and tooth decay
- D. kill germs
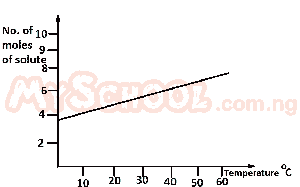
From the diagram above, find the amount of solute deposited when 200 cm\(^3\) of the solution is cooled from 55°C to 40°C.
- A. 0.10 mole
- B. 0.20mole
- C. 0.01 mole
- D. 0.02 mole
The arrangement of particles in crystal lattices can be studied using
- A. X - rays
- B. γ - rays
- C. α - rays
- D. β - rays
The molecular lattice of iodine is held together by
- A. dative bond
- B. metallic bond
- C. hydrogen bond
- D. van der Waal's forces
An isotope has an atomic number of 15 and a mass number of 31. The number of protons it contain is
- A. 16
- B. 15
- C. 46
- D. 31
The relative atomic mass of a naturally occurring lithium consisting of 90% \(^7 _3\)Li and \(^6 _3\)10% Li is
- A. 6.9
- B. 7.1
- C. 6.2
- D. 6.8
Which of the following questions is correct about the periodic table?
- A. The non-metallic properties of the elements tend to decrease across each period
- B. The valence electrons of the elements increase progressively across the period
- C. Elements in the same group have the same numbeer of electron shells
- D. Elements in the same period have the number of valence electrons
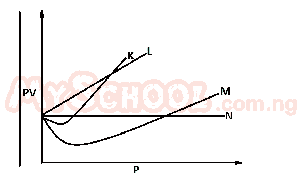
From the diagram above, an ideal can be represented by
- A. M
- B. N
- C. K
- D. L
Which of the following properties is NOT peculiar to matter?
- A. kinetic energy of particles increase from solid to gas
- B. Random motion of particles increases from liquid to gas
- C. Orderliness of particles increases from gas to liquid
- D. Random motion of particles increases from gas to solid


