Which of the following allotrope of carbon is a constituent of a lead pencil?
- A. Graphite
- B. Diamond
- C. Lampblack
- D. Soot
The gas that is most useful in protecting humans against solar radiation is?
- A. chlorine
- B. ozone
- C. carbon (IV) oxide
- D. hydrogen sulphide
The gasification of coke is used for the manufacturing of?
- A. producer gas
- B. natural gas
- C. synthetic gas
- D. industrial gas
Hydrogen sulphide gas can act as?
- A. an oxidizing agent
- B. a dehydrating agent
- C. a bleaching agent
- D. a precipitating agent
The oxidation of ammonia in excess air produces?
- A. NO
- B. N2O
- C. NO2
- D. N2O4
The equilibrium of an endothermic reaction which proceeds with an increase in volume can be shifted in the reverse direction by
- A. increasing the temperature and decreasing the pressure
- B. increasing the pressure and increasing the temperature
- C. decreasing the temperature and increasing the pressure
- D. decreasing the pressure and decreasing the temperature
CuO(s) + H\(_2\)(g) ↔ Cu(s) + H\(_2\)O(g)
What is the effect of increasing the pressure on the equilibrium reaction above?
- A. The equilibrium is shifted to the left
- B. The equilibrium is shifted to the right
- C. There is no effect
- D. More H2(g) is produced
A catalyst increase the rate of a chemical reaction by providing a path that?
- A. raise the activiation energy
- B. increase the temperature
- C. lowers the activation energy
- D. increase the concentration
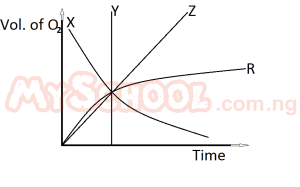
In the diagram above, which of the curves represents the evolution of oxygen with time in the equation 2KClO3(s) → 2KCl(s) + 3O2(g)?
- A. X
- B. Y
- C. Z
- D. R
Two equal bulbs, one containing ammonia and the other nitrogen are opened mouth – to – mouth to each other at room temperature. The entropy in the mixture of gases is likely to?
- A. remain unchange
- B. increase
- C. decrease
- D. change
C(s) + H2O(g) → H2(g) + CO2(g)
ΔG for the reaction above at 1300k is -43KJ. At this temperature, the reaction is?
- A. not feasible
- B. at equilibrium
- C. feasible
- D. exothermic
What current will deposit 3.25g of zinc in 2hrs?
[Zn = 65, F = 96500 mol–]
- A. 3.25A
- B. 2.00A
- C. 1.34A
- D. 0.67A
Which of the following metals is purified commercially by electrolysis?
- A. Zn
- B. Fe
- C. Sn
- D. Cu
The oxidation state of Cr in K\(_2\)Cr\(_2\)O\(_7\) is?
- A. +7
- B. +6
- C. +5
- D. 4
The colour changes observed when testing for reducing agents using acidified potassium heptaoxodichromate (VI) solution is?
- A. yellow to purple
- B. orange to green
- C. green to orange
- D. purple to yellow
The salt formed from a reaction between citric acid and sodium hydroxide in solution will be?
- A. acidic
- B. basic
- C. complex
- D. neutral
What volume of 1.5M solution of KOH would contain 0.045 moles
- A. 67.50 cm3
- B. 30.00 cm3
- C. 6.75 cm3
- D. 3.00 cm3
The acid used in electrolysis of water is dilute?
- A. HNO3
- B. CH3COOH
- C. H2SO4
- D. HCl
The major source of oxides of nitrogen is from the burning of
- A. coal
- B. wood
- C. fuel
- D. chlrofluorocarbons
For a given solute, the concentration of its saturated solution in different solvents are
- A. the same at the same temperature
- B. different at the same temperature
- C. the same at different temperature
- D. constant
If 10.5g of lead (II) trioxonitrate (V) is dissolved in 20cm3 of distilled water at 18oC, the solubility of the solute in mol dm-3 is
[Pb = 207, N = 14, O = 16]
- A. 1.60
- B. 5.25
- C. 16.00
- D. 525.00


