The uncovered raw food that is sold along major roads is likely to contain some amounts of
- A. Pb
- B. Cu
- C. Ag
- D. Na
117.0g of sodium chloride was dissolved in 1.0dm3 of distilled water at 25°C. Determine the solubility in mol dm-3 of sodium chloride at that temperature
[Na = 23, Cl = 35.5]
- A. 1.0
- B. 2.0
- C. 3.0
- D. 4.0
The vulcanizer’s solution is prepared by dissolving rubber in
- A. ethanol
- B. kerosine
- C. benzene
- D. petrol
When a few drops of water is added to a blue anhydrous cobalt (II) chloride, the colour changes to
- A. white
- B. pink
- C. red
- D. blue
In the course of purifying water for town supply the water is passed through large settlings tanks, containing sodium aluminate (III) to remove?
- A. large particles
- B. germs
- C. fine particles
- D. odour
Air boiled out of water as steam is richer in
- A. nitrogen and oxygen
- B. carbon (IV) oxide and oxygen
- C. noble gases and carbon (IV) oxide
- D. oxygen and noble gases
\( _{11} ^{23}Na + _0^1n → _{11}^{24}Na \)
The reaction above is an example of
- A. nuclear fission
- B. nuclear fusion
- C. artificial transmutation
- D. beta decay
In the periodic table, the electrical and thermal conductivities are properties of elements that
- A. decrease across the period and increase down the group
- B. increase across the period and decrease down the group
- C. decrease across the period and down the group`
- D. increase acrosss the period and down the group
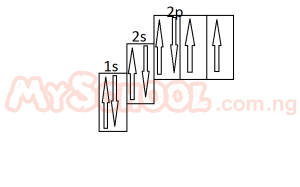
The diagram above represent the electron sub-level for
- A. carbon
- B. nitrogen
- C. oxygen
- D. flourine
The atom of an element X is represented as \(_z^yX\). The basic chemical properties of X depends on the value of
- A. Y
- B. Z
- C. Y - Z
- D. Z - Y
The experiment that showed that atoms have tiny positively charged nucleus was first carried out by
- A. Moseley
- B. Rutherford
- C. Millikan
- D. Dalton
Diffusion is slowest in solid particles than in particles of liquid and gases because
- A. solid particles have more kinetic energy than the particles of liquid and gases
- B. solid particles have less kinetic energy than the particles of liqiud and gases
- C. solid particles have less restriction in their movement than liquid and gas particles
- D. the particles in solids are far apart and the cohesive forces between them are negligible
300 cm3 of gas has a pressure of 800 mmHg. If the pressure is reduced to 650 mmHg, find its volume
- A. 243.75 cm3
- B. 369.23 cm3
- C. 738.46 cm3
- D. 1733.36 cm3
16.8 g of sodium hydrogen trioxocarbonate (IV) is completely decomposed by heat. Calculate the volume of carbon(IV) oxide given off at s.t.p
[Na 23, C = 12, O = 16, H = 1, Molar volume of a gas at s.t.p = 22.4 dm3]
- A. 22.40 dm3
- B. 11.20 dm3
- C. 2.24 dm3
- D. 1.12 dm3
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
From the equation above, calculate the mass of sodium hydroxide produced by 2.3g of sodium.
[H = 1, O = 16, Na = 23]
- A. 0.40g
- B. 0.80g
- C. 4.00g
- D. 8.00g
In countries where the temperature falls below 273K, salt is always sprinkled on the icy road in order to
- A. lower the melting point of the ice
- B. increase the density of the ice
- C. make the ice impure
- D. raise the melting point of the ice
Chlorophyll obtained from green leaves of plant can be shown to be composed of more than one coloured component by the technique of
- A. crystallization
- B. hydrolysis
- C. chromatography
- D. sublimation
What weight of NaCl is needed to make 2.0 dm3 of a 1.5M solution?
[Na = 23, Cl = 35.5]
- A. 116 g
- B. 175 g
- C. 87 g
- D. 58 g
Smog usually occurs over cities which
- A. burn a lot of fossil fuels
- B. have very few plants
- C. are surrounded by water
- D. are overpopulated
A good drying agent should be
- A. deliquescent
- B. effervescent
- C. hygroscopic
- D. efflorescent
The presence of nitrogen in air is to slow down
- A. respiration and combustion
- B. combustion and corrosion
- C. corrosion and respiration
- D. respiration and transpiration


