(a) If you were provided with anhydrous Na\(_2\)CO\(_3\), spatula and stirrer;
(i) list three other materials you would require to prepare a standard solution of Na\(_2\)CO\(_3\)
(ii) state what you would observe on adding diluted H\(_2\)SO\(_4\) to a portion of the Na\(_2\)CO\(_3\)
(b)(i) Describe briefly one chemical test you would perform to distinguish between zinc ions and aluminium ions in solution.
(ii) Mention one laboratory reagent you would use to;
I. produce ammonia from (NH\(_4\))\(_2\)SO\(_4\)
II. differentiate between precipitates of AgCl and Agl
lll. dehydrate ethanol
(c) Give the reason for each of the following laboratory practices
(i) Aqueous solutions of FeSO\(_4\) are freshly prepared when required for use.
(ii) The first jar of hydrogen collected during its preparation is discarded
Credit will be given for strict adherence to instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is one of the following substances; starch or sucrose or glucose D is a simple salt. Carry out the following exercises on C and D. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a)(i) Add about 5 cm\(^3\) of distilled water to a portion of C in a test tube. Stir thoroughly and test with litmus
(ii) Add about 2cm\(^2\) of Fehling’s solution to the resulting mixture from (a)(i) above the heat.
(b)(i) Heat a portion of D strongly in a test tube
(ii) Put the rest of D in a boiling tube and add about 10 cm\(^3\) of distilled water. Shake the mixture
(iii) Put about 2 cm\(^3\) of the mixture from (b)(ii)) in a test tube. Add aqueous ammonia in drops and then in excess
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but on no account of experiment procedure is required. All calculations must be done in your answer book.
A is mol dm HCI. B is a solution containing 15.0 g dm of a mixture of NaCl and KHCO\(_3\).
(a) Put A burette and titrate it against 20.0cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange as indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is: HCl\(_{aq}\) + KHCO\(_{3(aq)}\) \(\to\) KCl\(_{(aq)}\) +CO\(_{2(g)}\)
(b) From your results and the information provided above, calculate the:
(i) concentration of KHCO\(_3\), in mol dm\(^{-3}\) in B;
(ii) mass of KHCO\(_3\), in g dm\(^{-3}\) in B
(ii) Percentage by mass of KHCO\(_{3}\) in the mixture, [H=1; C = 12; O = 16; K = 39]
(iv) mass of NaCl in the mixture.
(a) Suggest how the following liquid reagents can be suitably stored in the laboratory
(i) X which fumes in moist air;
(ii) Y which is slowly decomposed by sunlight in ordinary reagent bottles.
(b) State what is observed when aqueous ammonia is added to:
(i) litmus paper;
(ii) Pb(NO\(_3\))\(_2\) solution in drops until in excess
(iii) freshly precipitated AgCI in excess.
(c) A salt sample was suspected to be either Na\(_2\)CO\(_2\) or NaHCO\(_3\). A student who was required to identify it tested a portion for solubility in water and then for effect on litmus paper.
(i) What was observed in each case?
(ii) Give the reason why the student’s procedure was unsuitable
(iii) Describe briefly how you would have identified the salt.
Credit will be given for strict adherence to the instructions, for observations precisely recorded and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the i.itne time they are made.
C is a sample of copper (II) tetraoxosulphate (VI) crystals. Carry out the following exercises on C. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a) Put half of C in a test tube and heat strongly
(b) Make solution of about 10 cm\(^{-3}\) with the sécond half of C and divide it into three portions
(i) To the first portion, add Sodium hydroxide solution in drops and then in excess. Heat the mixture
(ii) To the second portion, add aqueous ammonia in drops and then in excess followed by few drops of moderately concentrated HCI.
(iii) To the third portion, add all the zinc dust provided and stir thoroughly until there is a visible change.
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but no account or expeimental procedure is required. All calculations must be done in your answer book.
A is 0.125 mol dm\(^3\) H\(_2\)SO\(_4\). B is a solution containing X g dm\(^{-3}\) of NaOH.
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is H\(_2\)SO\(_4\) +2NaOH\(_{(aq)}\) \(\to\) Na\(_2\)SO\(_4\) + 2H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided above, calculate the;
(i) amount of H\(_2\)SO\(_4\) in the average volume of A used
(ii) Concentration of B in mol dm\(^{-3}\)
(iii) value of X.
[H = 1: O = 16; Na = 23]
(c) Describe briefly a suitable laboratory procedure for obtaining pure water from the titration mixture. (No diagram is required)
State what you would see on;
(i) bubbling SO\(_2\) into acidified KMnO\(_4\) solution
(ii) mixing zinc dust with CuSO\(_4\) solution
(iii) adding concentrated HNO\(_3\) to freshly prepared FeSO\(_4\) solution.
(b) List two substances in case which, if added to dilute H\(_2\)SO\(_4\), would give you
(i) H\(_{2(g)}\)
(ii) ZnSO\(_{4(aq)}\)
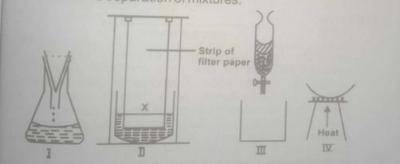
(c) The diagram labeled I to IV ABOVE illustrate different laboratory set-ups used in the separation of mixtures.
(i) Name the separation technique illustrated by each diagram
(ii) Which of the set-ups is used for concentrating dilute salt solutions, for the purpose of crystallization?
(iii) Which of the set-ups is used in obtaining clear water from muddy water?
(iv) Mention the set-up you would use to separate a polar solvent from a non-polar solvent
(v) State the modification you would make to the set-up labelled IV in order to use it for separating a mixture of NaCl and NH\(_4\)CI
Credit will be given for strict adherence to instructions, for observations precisely recorded, and for accurate interference. All tests, Observations and inferences must be clearly entered in your answer book, in ink at the time they are made. C and D are aqueous solutions of simple salt and an inorganic compound respectively. Carry out the following exercises on C and D. Record your observations and identify the gasses evolved. State the conclusion and draw from the result in the test and add sodium hydroxide in excess.
(b)(i) Test D with litmus paper
(ii) Put about 5cm\(^3\) of D in a test tube and add sodium hydroxide solution in drops and then in excess
(iii) Add about 1cm\(^3\) of the soap solution provided to about 10 cm\(^3\) of D mixture. Repeat the test using distilled water in place D
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer book. A is a solution of HCl containing 7.30g dm\(^{-3}\), B is a solution of X\(_2\)CO\(_{3}\) containing 10.6 gdm\(^{-3}\)
(i) Put A into your burette and titrate readings against 20.0 cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange as indicator. Tabulate your burette reading and calculate the average volume of A used. The equation for the reaction involved in the titration is ;
X\(_2\)CO\(_{3(aq)}\) + 2HCl\(_{(aq)}\) \(\to\) 2XCl\(_{(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
(ii) From your results and the information provided above, calculate the (i) concentration or A in mol dm\(^{3-}\)
Credit will be given for strict adherere to the instructions, for observations precisely recorded and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is a sample of iron (ii) tetraoxosulphate (VI). D is a sample of zinc trioxocarbonate (IV). Carry out the following exercises on C and D. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a)(i) Put all of C in a test tube and add about 5 cm\(^3\) of distilled water. Stir and test with litmus paper. Divide the solution into two portions
(ii) To the first portion, add sodium hydroxide solution in drops and then in excess.
(iii) To the second portion, add few drops of barium chloride solution, followed by dilute hydrochloric acid in excess.
(b)(i) Put half of D in a dry test tube and heat strongly. Allow to cool.
(ii) Add about 5 cm\(^3\) of dilute hydrochloric acid to the residue and divide the solution into two portions.
(iii) To the first portion, add sodium hydroxide solution in drops and then in excess.
(iv) To the second portion, add aqueous ammonia in drops and then in excess.
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A is a solution of HCI containing 5.0g dm\(^{-3}\). B is a solution of impure KOH containing 6.50g dm\(^{-3}\).
a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as indicator Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is: HCI\(_{(aq)}\) KOH\(_{(aq)}\) \(\to\) Cl\(_{(aq)}\) H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided above, calculate the:
(i) concentration of A in mol dm\(^{-3}\)
(ii) concentration of B in mol dm\(^{-3}\)
(iii) percentage purity of KOH in B [H= 1; CI = 35.5; KOH = 56.0g mol\(^{-1}\)]
(a) Give the reason in each case why it is necessary to:
(i) moisten a piece of litmus paper used in testing for the acidity or alkalinity of a gas.
(i) acidify the test solution with dilute hydrochloric acid in the confirmatory test for SO\(_4^2\)
(b)(i) List two gases that must not be prepared in the open laboratory
(ii) Mention one precaution that should be taken in the laboratory to prevent excessive inhalation of these gases during their preparation.
(iii) State one use of each of the following pieces of apparatus in the laboratory. I. Wash bottle II. Tripod stand
(c) State what would be observed when:
(i) Pb(NO\(_3\))\(_2\) is needed
(ii) concentrated HCI is added to MnO\(_2\)
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A is a solution of H\(_2\)SO\(_4\) containing 4.9 gdm-3, B is a solution containing X g dm\(^{-3}\) of Na\(_2\)CO\(_3\).
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as an indicator. Record the volume of your pipette. Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is; H\(_2\)SO\(_{4(aq)}\) + Na\(_2\)CO\(_{3(aq)}\) \(\to\) Na\(_{2}\)SO\(_{4(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
(b) From your results and information provided above, calculate the:
(i) Concentration of A In mol dm\(^{-3}\)
(ii) concentration of B in mol dm\(^{-3}\)
(iii) mass of salt formed when 500 cm\(^3\) of B is Completely neutralized by A.
(v) volume of carbon (IV) oxide liberated in (b) (ii) above at s.t.p. [O = 16, Na = 23, S = 32, 1 mole or a gas occupies 22.4 dm\(^3\) at s.t.p.]
Credit will be given for strict adherence to instructions, for observations precisely recorded, and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C Is an inorganic salt. D is an organic compound. Carry out the following exercises on C and D. Record your observations and identify any gases evolved. State the conclusion you draw from the result of each test.
(a) (i) Put C in a test tube and add about 10 cm\(^3\) of distilled water. Stir well.
(ii) Divide the resulting solution into two portions. To the first portion add sodium hydroxide solution in drops and then in excess.
(iii) To the second portion add dilute trioxonitrate (V) acid. Then add silver trioxonitrate (V) solution followed by aqueous ammonia in excess. tube and odd about 2- 5 cm\(^3\) of ‘Xn’.
(ii) Identify the functional group present in D.
(a) State what would be observed when:
(i) Chlorine is passed through a freshly prepared solution of FeCl\(_2\).
(ii) SO\(_2\) is bubbled into a solution of FeCl\(_3\);
(iii) A few drops of water Is added to sodium hydroxide pellets in a test tube;
(iv) Dilute H\(_2\)SO\(_4\) is added to CaCO\(_{3(s)}\)
(b) Three test tubes contain solutions of SO\(_3^{2-}\), CO\(^{2-}_3\) and SO\(_4^{2-}\) respectively. Describe one chemical method that you would use to identify the solution containing SO\(_4^{2-}\)
(c)(i) Draw and label a diagram to illustrate the separation of a mixture of petrol and water
(ii) Which of the following will dissolve faster? 10g of NaOH pellets in 100 cm\(^3\) of water; 10g of NaOH powder in 50 cm\(^3\) of water. Give the reason for your answer.
Burette reading (initial and final) must be given to two decimal places. Volume of pipette used must be recorded but no account of experimental procedure is required. All calculations must be done in you answer book.
A is a solution containing 6.3 g dm\(^{-3}\) of HNO\(_3\), B is a solution Na\(_2\)CO\(_3\)
(a) Put A Into the burette and titrate it against 20.0 cm\(^3\) portions of B using methyl orange indicator. Record the volume of your pipette. Repeat the titration to obtain consistent titres. Tabulate your burette readings and calculate the average volume of A used. The equation for the reaction involved in the titration is:
2HNO\(_{3(aq)}\) + Na\(_2\)CO\(_{3(aq)}\) \(\to\) 2NaNO\(_{3(aq)}\) + CO\(_{2(g)}\) + H\(_{2}\)O\(_{(l)}\)
(b) From your results and information provided above, calculate the;
(i) concentration of B in mol dm\(^{-3}\)
(ii) concentration of B in g dm\(^{-3}\):
(iii) mass of sodium ions in 1:0 dm\(^{-3}\) of B
[H = 1; C = 1; O = 16; N = 14; Na = 23]
Credit will be given for strict adherence to the instructions, for observations precisely recorded and accurate inferences. All tests, observations and inferences must be clearly entered in your als book, in ink, at the time they are made.
C is a mixture of an inorganic and organic compounds. Carry out the following exercises on C: Record your.observations and identity any gases evolved. State the conclusion you draw from the result of each test.
(a) Put all of C into a beaker and add 10 cm\(^3\) of distilled water: Stir the mixture thoroughly and filter. Keep both the filtrate and residue.
(b)(i) Test the filtrate with litmus paper.
(ii) To about 2 cm\(^3\) of the filtrate, add BaCI\(_{2(aq)}\) followed by dilute HCI:
(iii) To another 2 cm\(^3\) portion of the filtrate add NaOH and heat.
(c) Transfer the residue into a boiling tube and add few drops of iodine solution
(a) Consider the following compounds: MnO\(_2\), NaHCO\(_3\), Na\(_2\)CO\(_3\), NH\(_4\)CI cnd Pb(NO\(_3\))\(_2\) Select the compound(s) which;
(i) has a black colour
(ii) is a basic oxide:
(ii) sublime on heating
(iv) dissolves in water to give a solution of pH less than 7.
(b) State the colour of each af the following aqueous Solutions:
(i) Calcium hydroxide;
(ii) iron (III) trioxonitrate (V):
(ii) Copper (II) tetraoxosulphate (VI);
(iv) Poassium heptaoxodichromate (VI).
(c) Give one example of a neutral oxide which is a colourless liquid at room temperature.
(d) Draw and label a diagram for a set-up that can be used for the separation of two immiscible liquids
All your burette readings (initial and final) as well as the size size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer book.
A is a solution containing 1.04 g HCl per 500 cm\(^3\) of solution. B was prepared by diluting 50.0 cm\(^3\) of a saturated solution of Na\(2\)CO\(_3\) at room temperature to 1000 cm\(^3\)
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^{3}\) portions of B using methyl orange as indicator. Repeat the titration to obtain consistent titres. Tabulate your results and calculate the average volume of acid used.
9b) From your results and information provided above, calculate the;
(i) concentration of A in moldm\(^{-3}\)
(ii) concentration of B in mol dm\(^{-3}\)
(iii) solubility of Na\(_2\)CO\(_3\) in mol dm\(^{-3}\)
(iv) volume of CO\(_2\) that would be liberated from 1 dm\(^3\) of B if the titration were carried out at s.t.p.
The equation for the reaction is Na\(_2\)CO\(_{3(aq)}\) + 2HCl\(_{(aq)}\) \(\to\) 2NaCl\(_{(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
[H = 1; C = 12; O = 16; Na = 23; Cl = 35.5; Molar volume of gas at s.t.p = 22.4 dm\(^3\)]
Credit will be given for strict adherence to the instructions, for observations precisely recorded, and for accurate inferences. All tests, Observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C is a mixture of two inorganic salts. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) Put all of C in a test tube and add about 10 cm\(^3\) of distilled water. Stir, filter, and keep the filtrate and the residue.
(b) Put the residue into a test tube and add about 5 cm\(^3\) of dilute HCl. Shake to dissolve
(i) To about 2 cm\(^3\) of the solution, add NaOH\(_{(aq)}\) in drops and then in excess
(ii) To another 2 cm\(^3\) portion of the solution, add NH\(_{3(aq)}\) in drops and then in excess.
(c) To about 2 cm\(^3\) portion of the filtrate, add few drops of dilute HNO\(_3\), and then AgNO\(_{3(aq)}\) a followed by aqueous NH\(_3\) in excess.
(a) State an indicator suitable for the titration of;
(i) dilute HCl and NaOH\(_{3(aq)}\)
(ii) dilute CH\(_3\)COOH and KOH\(_{(aq)}\)
(iii) dilute HCl and NH\(_{3(aq)}\).
Give a reason for your answer in each case.
(b) Calculate the volume of water that would be added to 50 cm\(^3\) of 0.10 mol dm\(^{-3}\) of HCI to dilute it to 0.010 mol dm \(^{-3}\)
(c) Name one gas that could be used to demonstrate the fountain experiment.

