All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account experimental procedure is required. All calculations must be done in your answer booklet.
A is 0.100 mol dm\(^{-3}\) HNO\(_3\). B is a solution containing 2.50 g of a mixture of Na\(_2\)CO\(_3\) and Na\(_2\)SO\(_4\) in 250 cm\(^3\) of solution.
(a) Put into the burette and titrate it against 20.0 cm\(^3\) or 25.0cm\(^3\) portions of B using methyl orange as indicator. Repeat the exercise to obtain consistent titre values. Tabulate your readings and calculate the average volume of acid A used. The equation of reaction is Na\(_2\)CO\(_{3(aq)}\) + 2HNO\(_{3(aq)}\) \(\to\) NaNO\(_{3(aq)}\) + H\(_2\)O\(_{(l)}\) + CO\(_{2(g)}\)
(b) From your results and the information provided, calculate the:
(i) concentration of B in moldm\(^{-3}\);
(ii) concentration of Na\(_2\)CO\(_3\) in B in gdm\(^{-3}\);
(iii) percentage of Na\(_2\)CO\(_3\), in the mixture. [Na\(_2\)CO\(_3\) = 106]
Credit will be given for strict adherence to the instructions, for observations precisely recorded and for accurate inferences. All tests, observations, and inferences must be clearly entered in your answer book, in ink, at the time they are made.
C and D are inorganic salts. E is a solution of an organic compound. Carry out the following exercises on C, D and E. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) To all of C, add about 2 cm\(^3\) of distilled water in a boiling tube. Shake to dissolve and test the solution with litmus paper.
(b)i) Test E with litmus paper.
(ii) Add all of E to the solution obtained in (a).
(c)(i) Add about 5 cm\(^3\) of distilled water to D in a test tube and shake thoroughly. Divide the resulting solution into two portions.
(ii) To the first portion, add few drops of BaCl\(_{2(aq)}\) followed by excess dil. HCl.
(iii) To the second portion, add HNO\(_{3(aq)}\) in drops and then in excess.
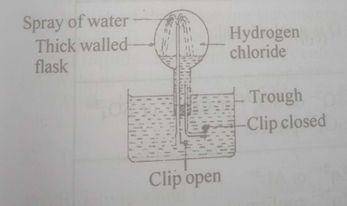
(a) Consider the following diagram ABOVE.
(i) Give the name of the experiment.
(ii) What does the experiment demonstrate?
(iii) Name one gas that could be used in place of HCl gas.
(iv) What colour could be observed in the flask during the spray of water?
(v) Could the gases used in the experiment be collected over water?
(vi) Explain briefly your answer in (a)(v).
(b) A solid substance U when strongly heated decomposes to give a white solid V and carbon (IV) oxide. When water is added to V, W is produced W can be used to test for carbon (IV) Oxide. Identify U, V and W.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet
A solution of 0.050 moldm\(^3\) H\(_2\)C\(_2\)O\(_4\) (ethanedioic acid). B is a solution of KMnO\(_4\), (potassium tetraoxomanganate (VII), of unknown concentration.
(a) Put B into the burette. Pipette 20.0 cm\(^3\) or 25.0 cm\(^3\) of A into a Conical flask and add about 10.0 cm\(^3\) of dilute H\(_2\)SO\(_4\), Heat the mixture to about 40°C – 50°C and titrate it while still hot with B. Repeat the titration to obtain consistent titre values. Tabulate your results and calculate the average volume of B used. The equation of reaction is;
2MnO\(_{4(aq)}^-\) + 5C\(_2\)O\(_{4(aq)}^{2-}\) + 16H\(^+_{(aq)}\) \(\to\) 2MnH\(^{2+}_{(aq)}\) + 8H\(_2\)O\(_{(l)}\) + 10CO\(_{2(g)}\)
(b) From your results and the information provided, calculate the:
(i) concentration of MnO\(_2^-\) in B in moldm\(^{-1}\)
(ii) concentration of KMnO\(_4^-\) in B in gdm\(^{-3}\)
(iii) volume of CO\(_2\) evolved at s.t.p when 25.0 cm\(^3\) of H\(_2\)C\(_2\)O\(_4\) reacted completely. [0 = 16.0, K= 39.0, Mn = 55.0, Molar volume of gas at s.t.p.= 22.4 dm\(^3\) mol\(^{-1}\)]
Credit will be given for strict adherence to the instructions. for observations precisely recorded and jor accurate inferences. All tests, observations and inferences must be clearly entered in your answer book in ink, at the time they are made
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet
C is a mıxture of two salts, containing one cation and two anions. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusion you draw from the result of each test.
(a) Put all of C in a beaker and add about 10 cm\(^3\) of distilled water. Stir well and filter. Keep the filtrate and the residue.
(b) To about 2 cm\(^3\) of the filtrate, add few drops of AgNO\(_{(aq)}\), followed by HNO\(_{3(g)}\). Add excess NH\(_{3(aq)}\) to the resulting mixture.
(c)(i) Put all of the residue into a clean test tube and add about 5 cm of HNO\(_{3(aq)}\)
(ii) To about 2 cm\(^3\) of the solution from 2(c)(i), add NaOH\(_{(aq)}\) in drops and then in excess
(iii) To another 2 cm\(^3\) of the solution from 2(c)(i), add NH\(_{3(aq)}\) in drops and then in excess.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet
(a) What difference in physical properties enable the separation of mixtures by:
(i) simple distillation,
(ii) paper chromatography;
(iii) fractional distillation.
(b) Give a reason for each of the following practices during titration in the laboratory.
(i) White tile is placed under the conical flask.
(ii) Burette readings are always recorded to two decimal places.
(iii) Calculate the volume of 2.5 moldm\(^{-3}\) stock HČI required to prepare 500 cm\(^3\) of 0.20 moldm HCI.
All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
A is 0.200 moldm\(^3\) of HCl. C is a solution containing 14.3g of Na2CO\(_{3}\).xH\(_2\)O in 500 cm\(^3\) of solution.
a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0cm\(^3\) portions of C using methyl orange as indicator. Repeat the titration to obtain Consistent titre values. Tabulate vour results and calculate the average volume of A used. The equation for the reaction is;
Na\(_2\)CO\(_3\) . xH\(_2\)O + 2HCl\(_{(aq)}\) \(\to\) 2NaCl\(_{(aq)}\) + CO\(_{2(g)}\) + (x + 1)\(_3\)H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided. calculate the:
(i) concentration of C in moldm\(^{-3}\)
(ii) concentration of C in gdm\(^{-3}\)
(iii) molar mass of Na\(_2\)CO\(_3\) . xH\(_2\)O
(iv) the value of x in Na\(_2\)CO\(_3\) . xH\(_2\)O. [H =1.0; C = 12.0; O = 16.0; Na = 23.0]
Credit will be given for strict adherence to the instruction, for observations precisely recorded and for accurale references. All tests. obsenations and influences must be cleary entered in the booklet in ink at the same time they are made.
All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
F is 2 mixture of two inorganic salts. Carry out the following exercises on F. Record your observations and identify any gas(es) evolved. State the conclusions you draw from the result of each test.
(a) Put all of F in a beaker and add about 10 cm\(^3\) of distilled water. Stir well and filter. Keep the filtrate and the residue.
(b)(i) To about 2cm\(^3\) of the filtrate. add NaOH\(_{(aq)}\) in drops and then in excess.
(ii) To another 2cm\(^3\) portion of the solution, add a few drops of NH3\(_{(aq)}\) in drops and then in excess.
(c) To about 2cm\(^3\) of the solution, add a few drops of HNO\(_{3(aq)}\) followed by few drops of the drops of AgNO\(_{3(aq)}\)
(d)(i) Put all the residue into a clean test-tube and add HNO\(_{3(aq)}\)
(ii) To a portion of the solution from (d)(i)) add NaOH\(_{(aq)}\) in drops and then in excess.
All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
State what would be observed if the following reactions are carried out in the laboratory:
(i) methyl orange is dropped into a solution of lime juice:
(ii) hydrogen sulphide gas is bubbled through Iron (III) chloride solution:
(iii) sulphur (IV) oxide gas is bubbled into acidified solution of KMnO\(_4\):
(iv) ethanoic acid is added to a solution of Ka\(_2\)CO\(_3\)
All your burette readings (initials and final) as well as the size of your pipette must he recorded but no account of experimental procedure is required. All calculations must be done in your booklet.
State the observation that would be made when each of the following reactions is carried out in the laboratory:
(a) Addition of 2 cm\(^3\) of bench H\(_2\)SO\(_{4(aq)}\) to 2 cm\(^{3}\) of barium chloride solution;
(b) Addition of 2 cm\(^3\) of dilute hydrochloric acid to 1g of powered iron(II) sulphide (FeS):
(C) Addition of 2 cm\(^3\) of dilute hydrochloric acid to 1g of iron filings and allowed to stand for sometime.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your booklet.
C and D are inorganic salts. Carry out the following exercises on them. Record your observations and identify any gas(es) evolved. State the conclusions you draw from the result of each test.
(a) Put all of C in a test tube and add about 5 cm of distilled water. Shake thoroughly and test the resulting solution with Litmus paper. Divide the solution into three portions.
(i) To the first portion, add NaOH\(_{(aq)}\) in drops, then in excess.
(ii) To the second portion. add NH\(_{3(aq)}\) in drops. then in excess.
(iii) To the third portion. add AgNO\(_{3 (aq)}\) followed by HCl\(_{(aq)}\)
(b)(i) Put all of D in a test tube and add about 5 cm\(^3\) of distilled water. Shakę thoroughly and feel the test tube.
(ii) To about 2 cm\(^3\) of the solution, add HCl\(_{(aq)}\)
All your burette readings (initials and final) as well as the size of your pipette must he recorded but no account of experimental procedure is required. All calculations must be done in your booklet.
A solution of potassium tetraoxomanganate( VII). B is a solution of iron(II)chloride containing 4.80g of the salt in 250 cm\(^{3}\) of solution.
(a) Put A into the burette. Pipette 20.0cm\(^3\) or 2.50.0 of B into a conical flask, add 20.0 cm\(^3\) of H\(_2\)SO4\(_{(aq)}\) and titrate with A. Repeat the titration to obtain concordant titre values. Tabulate your results and calculate the average volume of A used. The equation of the reaction is: MnO\(_{4(aq)}\) + 5Fe\(^{3+}_{ (aq)}\) + H\(_2\))
(b) From your results and the information provided, calculate the;
(i) concentration of B moldm\(^{-3}\):
(ii) concentration of A in moldm\(^{-3}\)
(iii) number of moles of Fe\(^{2+}\) in the volume of B pipetted. [FeCl\(_2\) = 127 gmol\(^{-1}\)] Credit will be given for strict adherence to the instruction, for observations precisely recorded and for accurate inferences. AlI tests, Observations and inferences must be clearly entered in the booklet in ink at the time they are made.
All your burette readings (initials and final), as well as the size of your pipette, must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
(a) State what would be observed when \(BaCl_2\) solution is a portion of a saturated Na\(_2\)CO\(_2\) followed by dilute HCI in excess.
(ii) A gas Q decolorized acidified kMnO\(_4\) Solution. Suggest what Q could be
(b) Name one substance used in the laboratory for drying each of the following substances:
(i) ammonia gas:
(ii) carbon (IV) oxide.
(c) Give a reason why a given mass of sodium hydroxide pellets cannot be used to prepare a standard solution.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
C is a mixture of two inorganic compounds. Carry out the following exercises on C. Record your observations and identify any gas(es) evolved. State the conclusions you draw from the result of each test.
(a) Put all of C in a boiling tube and add about 10 cm\(^3\) of distilled water. Shake thoroughly and filter. Keep both the residue and the filtrate.
(b)(i) To about 2 cm\(^3\) of the filtrate add a few drops of Silver trioxoitrate (V) followed by dilute HNO\(_{3}\)
(ii) Add excess NH\(_3\) solution to the resulting mixture in (b)(i).
(C)(i) Put the residue in a test tube, add about 2 cm\(^3\) of dilute HCl and shake.
(ii) Add NH\(_3\) Solution in drops to the mixture from (c)(i) and then in excess.
All your burette readings (initials and final) as well as the size of your pipette must be recorded but no account of experimental procedure is required. All calculations must be done in your answer booklet.
A is a solution containing 15.8 g dm\(^3\) of Na\(_2\)S\(_2\)O\(_3\). B was obtained by dissolving 9.0 g of an impure sample of I\(_2\) in aqueous Kl and the solution made up to 1 dm\(^3\).
(a) Put A into the burette and titrate it against 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B. Use starch solution as indicator. Repeat the titration to obtain concordant titre values. Tabulate your results and calculate the average volume of A used. The equation for the reaction involved in the titration is I\(_2\) + 2S\(_2\)O\(_3\) \(\to\) 2I\(^-\) + S\(_4\)O\(_6^{2-}\).
(b) From your results and the information provided, calculate the:
(i) concentration of A in mol dm\(^{-3}\)
(ii) concentration of I\(_2\) in B in mol dm\(^{-3}\);
(iii) percentage by mass of I\(_2\) in the sample
(c) Give reasons why the starch indicator was not added to the titration mixture at the beginning of the titration. [O = 16.0, Na = 23.0, S = 32.0, 1 = 127.0] Credit will be given for strict adherence to the instructions for observations precisely recorded and for accurate inferences. AIl tests, observations and inferences must be clearly entered in the booklet in ink at the time they are made.
(a) A zinc salt, E when heated strongly, produced a brown gas with pungent smell, a colourless gas that rekindled a glowing splint, and a residue that was allowed to cool.
(i) identify the salt E.
(ii) Write an equation for the decomposition of E.
(iii) State what would be observed when the residue was allowed to cool.
(b) Describe how 250cm\(^3\) of 0.2 mol dm\(^3\) H\(_2\)SO\(_4\) could be prepared from 150 cm\(^3\) of a 1.0 mol dm\(^{3}\) stock solution of the acid.
(c) State the effect of aqueous solution of Al\(_2(SO_4)_3\) on litmus paper.
C is an organic compound. Carry out the following exercises on C. Record your observations and identity any gas(es) evolved. State the conclusions you draw from the results of each test.
(a) Put about 10 drops of C on a watch glass and ignite it using a burning splint.
(b)(i) Put about 1 cm\(^3\) of C in a test tube and add about 1 cm\(^3\) of distilled water. Shake the test tube.
(ii) Put about 1 cm\(^3\) of C in a test tube and add about 2 cm\(^3\) of acidified K\(_2\)Cr\(_2\)O\(_7\) solution. Warm the mixture gently and leave to stand for 5 minutes.
(c) Put few crystals of specimen D in a test tube and add about 2cm\(^3\) of C followed by about 2 cm\(^3\) of 10 % NaOH\(_{(aq)}\) Shake the test tube vigorously.
(d) State the class of compounds to which C belongs.
All your burette readings (initial and final), as well as the size of your pipette, must be recorded but no account of the experimental procedure is required. All calculations must be done in your answer booklet.
A is a solution containing 5.00 g of HNO\(_3\) in 500 cm\(^3) of solution. B is a solution of NaOH of unknown concentration.
(a) Put A into the burette and titrate it with 20.0 cm\(^3\) or 25.0 cm\(^3\) portions of B using methyl orange as an indicator. Repeat the titration to obtain concordant titre values. Tabulate your results and calculate the average volume of acid used. Equation of the reaction is HNO\(_{3(aq)}\) + NaOH\(_{(aq)}\) \(\to\) NaNO\(_{3(aq)}\) + H\(_2\)O\(_{(l)}\)
(b) From your results and the information provided. calculate the: (i) concentration ot A In mol dm\(^{-3}\)
(ii) concentration of B in mol dm\(^{-3}\).
(iii) concentration of B in gdm\(^{-3}\)
(iv) mass of NaNO\(_3\) formed. If 250 cm\(^3\) of NaOH were neutralised. [Molar mass of NaOH = 40g mol\(^{-1}\), NaNO\(_3\) = 85 gmol\(^{-1}\). Credit will be given for strict adherence to the instructions. for observations precisely recorded and for accurate inferences. All tests, observations and inferences must be clearly entered in this booklet, in ink, at the time they are made.
(a)(i) Draw and label a diagram to illustrate the preparation and collection of dry chlorine gas in the laboratory.
(ii) State two uses of chlorine.
(b) Describe the preparation of hydrogen from water gas.
(i) Name the chief ore of aluminium.
(ii) Why is the ore purified?
(iii) Name the electrode used in the electrolysis.
(iv) Give one reason why cryolite, NaAlF\(_6\), is added to the electrolyte.
(c) Name three products obtained directly from the destructive distillation of coal.
(a) The following reaction scheme is an illustration of the contact process. Study the scheme and answer the questions that follow.
(i) Name X and Y
(ii) Write a balanced chemical equation for each of the processes I, II, III and IV
(iii) Name the catalyst used in process II
(iv) Using Le Chatelier’s principle, explain briefly why increasing the temperature would not favour the reaction in II
(v) State two uses of SO\(_2\)
(b) Consider the following equation: 2H\(_{2(g)}\) + O\(_{2(g)}\) \(\to\) 2H\(_2\)O\(_{(g)}\)
Calculate the volume of unused oxygen gas when 40 cm\(^3\) of hydrogen gas is sparked with 30cm\(^3\) of oxygen gas
(c) Calcium carbonate of mass 1.0 g was heated until there was no further change.
- Write an equation for the reaction which took place.
- Calculate the mass of the residue.
- Calculate the volume of the gas evolved at s.t.p.
- What would be the volume of the gas measured at 15 and 760 mm Hg? [C= 12.0, O = 16.0, Ca = 40.0, molar volume of a gas at s.t.p. = 22.4 dm\(^3\) ]
(a) In the Solvay process, explain briefly with equations the functions of the following substances:
- limestone;
- ammonia.
(b) (i) Write a chemical equation for the fermentation of glucose.
(ii) Explain briefly why a tightly-corked glass bottle filled to the brim with fresh palm-wine shatters on standing for some time.
(c) Consider the following metals: Na, Fe, K and Cu.
(i) Arrange the metals in order of increasing reactivity.
(ii) Which of the metals will react with cold water?
(ii) Which of the metals could form coloured salts?
(d)(i) What is a redox reaction?
(ii) Identify which of the following reaction equations are redox.
(I) 2Na + Cl\(_2\) → 2NaCl
(II) AgCl + 2NH\(_3\) → [Ag(NH\(_3\))\(_2\)]Cl
(III) C\(_2\)H\(_2\) + H\(_2\) → C\(_2\)H\(_4\)
(IV) HCl + KOH → KCl + H\(_2\)O
(V) 2FeCl\(_3\) + 2KI → 2FeCl\(_2\) + 2KCl + I\(_2\)
(iii) Give a reason for each of the answers in (d)(ii).
(iv) Write balanced equations of the half reactions for any two of the redox reactions in (d)(ii).

