The following molecules have double covalent bonds between two atoms except?
- A. oxygen
- B. carbon (IV)oxide
- C. ethene
- D. water
Which of the following solids would not decompose on heating?
- A. Ammonium chloride
- B. Lead (i) trioxonitrate (V)
- C. Potassium trioxocarbonate (IV)
- D. Sodium hydrogen trioxocarbonate (IV)
The boiling point of pentane is higher than that of propane because?
- A. carbon-carbon single bonds are stronger than carbon-hydrogen bonds.
- B. Pentane has more covalent bonds to break
- C. Pentane does not burn easily as propane
- D. The intermolecular forces in pentane are stronger than those of propane
A factor that is considered most important when siting a chemical industry is?
- A. nearness to other industrial establishments
- B. nearness to raw materials
- C. favourable climate conditions
- D. availability of storage facilities
Which of the following pairs of elements has the greatest difference in electronegativity?
- A. Na and F
- B. Na and CI
- C. Na and Br
- D. Na and I
A balanced chemical equation is based on the law of?
- A. Periodicity
- B. Constant composition
- C. Multiple proportion
- D. Conservation of mass
The oxidation state of chlorine in NaClO\(_3\) is?
- A. +1
- B. +3
- C. +5
- D. +6
When an ionic bond is broken, bonding electrons are
- A. shared between participating atoms
- B. gained by the most electropositive atom
- C. gained by the most electronegative atom
- D. lost by both participating atoms.
Group VIl elements in their combined states are called?
- A. Halogens
- B. Anions
- C. Halide
- D. Cations
The most suitable process of obtaining water from an aqueous solution of sugar i
- A. Crystallization
- B. Distillation
- C. Filtration
- D. Decantation
Which of the following pairs of properties of alkali metals decreases down the group?
- A. First ionization energy and reactivity
- B. melting point and atomic radius
- C. Reactivity and electronegativity
- D. First ionization energy and melting point
The high solubility of ethanol in water is due to?
- A. its low boiling point.
- B. its low freezing point.
- C. its covalent nature
- D. hydrogen bonding
Equal masses of calcium trioxocarbonate(iv) were added to dilute hydrochloric acid at the temperature specified. Under which of the following conditions would the reaction be slowest?
- A. Calcium trioxocarbonate ( iv ) chips a 20°C
- B. CalcIum trioxocarbonate (IV) chips at 40°C
- C. Calcium trioxocarbonate (IV) powder at 20'C
- D. Calcium trioxocarbonate (IV) powder at 40'C
What is the mass of silver deposited when 24,125 C of electricity is passed through a solution or silver salt.
[Ag = 108, IF = 96,500 C ]
- A. 432g
- B. 108g
- C. 54g
- D. 27g
What quantity of electrons is lost when one mole of iron (II) ions is oxidized to iron (III) ?
- A. 0 mole
- B. 3 moles
- C. I mole
- D. 2 moles
Which or the following products could be formed during incomplete combustion of a hydrocarbon.
i.Carbon ii. Hydrogen III. Carbon (Il) oxide
- A. I only
- B. I and II only
- C. I and III only
- D. II and III only
Which or the following substances is a polypeptide?
- A. Starch
- B. Glycogen
- C. Protein
- D. Fats
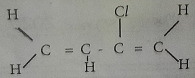
The IUPAC name of the compound represented by the structure below is ?
- A. 2- chloro but-I ,3-diene.
- B. But-1,3-chlorodiene.
- C. 2-chloro but-diene.
- D. 3 -chloro but -1.3-diene.
Which of the following substances is an example of a fine chemical?
- A. Sodium hydroxide
- B. Hydrochloric acid
- C. Ethylene
- D. Ammonia
Kipps apparatus can be used to prepare?
- A. H\(_2\)
- B. NH\(_3\)
- C. O\(_2\)
- D. H\(_2\)S
The group to which elements belong in the periodic table is determined by the number?
- A. electrons
- B. core electrons
- C. valence shells
- D. valence electrons


