32g of anhydrous copper(ii)tetraoxosulphate(vi) dissolved in 1dm\(^3\) of water generated 13.0kj of heat. The heat solution is?
- A. 26.0kj/mol
- B. 65.0kj/mol
- C. 130.0kj/mol
- D. 260.0kj/mol
Helium is used in observation of ballons because it is?
- A. Light and combustible
- B. light and non-combustible
- C. heavy and combustible
- D. heavy and non-combustible
Deliquescent substances are also?
- A. efflorescent
- B. anhydrous
- C. hygroscopic
- D. insoluble
Chromatography is used to separate components of mixture which differ in their rates of ?
- A. diffusion
- B. migration
- C. reaction
- D. sedimentation
A few drop of conc HCL are added to about 10cm\(^3\) of a solution of PH 3.4. The PH of the resulting mixture is?
- A. less than 3.4
- B. greater than 3.4
- C. unaltered
- D. the same as that of the pure water
The shape of ammonia molecule is?
- A. Trigonal planar
- B. Octahedral
- C. Square planar
- D. Tetrahedral
Which of the following gases will rekindle a brightly splint?
- A. NO\(_2\)
- B. NO
- C. N\(_2\)O
- D. CL\(_2\)
The volume occupied by 1.58g of a gas at S.T.P is 500cm\(^3\). What is the relative molecular mass of the gas? [ G.M.V at S.T.P = 22.4dm\(^{3}\) ]
- A. 28
- B. 32
- C. 44
- D. 71
The number of molecules of Carbon(iv)Oxide produced when 10.0g of CaCO\(_3\) is treated with 0.2dm\(^3\) of 1 Mole of HCL in the equation
CaCO\(_3\) + 2HCL ⇒ CaCl\(_2\) + H\(_2\) O + CO\(_2\) , is?
- A. 1.00 X 10\(^{23}\)
- B. 6.02 X 10\(^{23}\)
- C. 6.02 X 10\(^{22}\)
- D. 6.02 X 10\(^{24}\)
How many valence electrons are contained in the element \(^{31}_{15}P\)?
- A. 3
- B. 4
- C. 15
- D. 31
The products of the thermal decomposition of ammonium trioxonitrate(v) are?
- A. Nitrogen (I) Oxide and Water
- B. Ammonia and Oxide
- C. Nitrogen and Water
- D. Nitrogen (IV) Oxide and Water
Four elements P, Q, R and S have atomic numbers of 4, 10, 12 and 14 respectively. Which of these elements is noble gas?
- A. P
- B. Q
- C. R
- D. S
If the quantity of oxygen occupying 2.76L container at a pressure of 0.825 atm and 300k is reduced by one-half, what is the pressure exerted by the remaining gas?
- A. 1.650atm
- B. 0.825atm
- C. 0.413atm
- D. 0.275atm
Which of the following substances is a mixture?
- A. Granulated sugar
- B. Seawater
- C. Sodium chloride
- D. Iron filling
When water drops are added to calcium carbide in a container and the gas produced is passed through a jet and lighted, the resultant flame is called an?
- A. Oxyethene
- B. Oxyhydrocarbon flame
- C. Oxyacetylene flame
- D. Oxymethane flame
Liquid black soap is made by boiling palm oil with liquid extract of ash. The function of the ash is to provide the ?
- A. Acid
- B. Ester of alkanoic acid
- C. Alkali
- D. Alkanol
What process would coal undergo to give coal gas, coal tar, ammoniacal liquor and coke?
- A. Steam distillation
- B. Destructive distillation
- C. Liquefication
- D. Hydrolysis
What are the possible oxidation numbers of an element if its atomic number is 17?
- A. -1 and 7
- B. -1 and 6
- C. -3 and 5
- D. -2 and 6
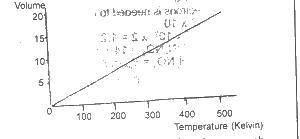
Which of the gas laws does this graph illustrate?
- A. Boyle
- B. Charles
- C. Gay-Lussac
- D. Graham
The best treatment for a student who accidentally poured conc tetraoxosulphate(vi) on his skin in the laboratory is to wash his skin with?
- A. with cool running water
- B. sodium hydroxide solution
- C. iodine solution
- D. sodium trioxonitrate(v) solution
8g of CH\(_4\) occupies 11.2 at S.T.P. What volume would 22g of CH\(_3\)CH\(_2\)CH\(_3\) occupy under the same condition?
- A. 3.7dm\(^3\)
- B. 11.2dm\(^3\)
- C. 22.4dm\(^3\)
- D. 33dm\(^3\)


