(a)(i) Distinguish between a strong acid and a concentrated acid.
(ii) What is meant by amphoteric oxide? Give one example.
(b)(i) Describe the manufacture of tetraoxosulphate (VI) acid by contact process.
(ii) Write one equation each to show the action of tetraoxosulphate (VI) acid respectively as a dehydrating agent and as an oxidizing agent.
(iii) Give the reason why tetraoxosulphate classified as a heavy chemical.
The compound whose formula is written below is a major component of a soft fatty substance:
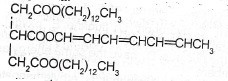
(a) State the change that would be observed in the physical state of the fatty substance if hydrogen were bubbled through it for long time in the presence of finely divided nickel at about 180\(^o\)C
(c) Determine the amount (in mole) of hydrogen that would be consumed if one mole of the component reacted completely with hydrogen.
(c) State the product of the reaction of the fatty substance with hot concentrated sodium hydroxide solution
(a) X and Y belong to the same period in the Periodic Table is a group I element while Y belongs to group VII. State which of the elements would
(i) be a good oxidizing agent
(ii) have the smaller atomic volume
(iii) have the higher ionization potential
(b) Explain your answer in (a)(i) above.
State which of the following can exhibit geometric isomerism:
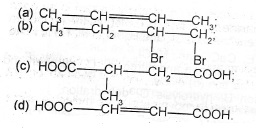
Give reason for your answer
Mention the respective properties of the following allotropes of carbon that account for their uses as indicated:
(a) diamond used for drilling rocks;
(b) diamond used as jewels;
(c) graphite used as electrodes;
(d) graphite used for slowing down neutrons in nuclear reactors;
(e) wood charcoal used in gas masks.
(a) Name the residue obtained on strongly heating the following:
(i) ZnCO\(_3\) in an open crucible;
(ii) CuSO\(_4\), 5H\(_2\)O and then allowing it to cool in a desiccator.
(b) State the colour changes observed on heating and cooling in each case in(a) above.
(a) State the phenomenon illustrated by the:
(i) spreading of the smell of hydrogen sulphide gas in the laboratory;
(ii) existence of atoms of the same element having different mass numbers
(b) The atomic number of an element is 17. It has different atoms containing 18 neutrons and 20 neutrons, with a relative abundance of 75% and 25% respectively. Calculate the relative atomic mass of the element.
(a) If L is the Avogadro constant and E° is standard cell potential, state what X and Y stand for in the following expressions
(i) X = \(\frac{\text{Mass of L molecules of gas or vapour}}{\text{ Mass of L molecules of hydrogen}}\)
(ii) Y = -nFE°
(b) State two differences between a primary cell and a secondary cell.
(a) State one air pollution that causes:
(i) blood poisoning
(ii) acid poisoning
(iii) blackening of the walls of buildings
(b) Mention one major chemical industry in each case which requires the following as raw materials:
(i) petrochemicals;
(ii) cellulose.
(a) Give one disadvantage of:
(i) hard water
(ii) soft water
(b) Explain why the degree of hardness in a sample of clear lime water is higher than in another sample has that been turned milky by carbon (IV) oxide.
Classify each of the following as physical change or a chemical change:
(a) fractional distillation of liquefied air;
(b) cracking of petroleum fractions;
(c) conversion of rhombic sulphur to monoclinic sulphur;
(d) chromatographic separation of chlorophyll.
(a) (i) Define heat of combustion.
(ii) What name is given to the container used for determining tne reaction?
(b) The heat of combustion of carbon in excess air is – 3935 kJ.
(i) Sketch an energy profile diagram for the reaction.
(ii) Calculate the heat change when 60 g of carbon undergoes complete combustion to produce carbon (IV) oxide. (C = 12)
(iii) Explain why the value of the heat of neutralization of strong acids by strong bases is constant.
(c) Give reason for the following:
(i) rusting of iron is regarded as a slow combustion process;
(ii) iron filings rust much more faster than iron nails when exposed to the same atmospheric condition;
(iii) iron is better protected from corrosion by plating it with.zinc than with tin
(a)(i) Determine the maximum number of electrons that can occupy the principal energy level M of an atom.
(ii) Show the changes in the electronic structures of atoms of sodium and fluorine (\(^{23}_{11}\)Na: \(^{19}_{9}F\)) when they combine to form sodium fluoride.
(iii) State: three properties that sodium fluoride would have, based a-n the bond type present in the compound.
(b) Write equations to show when:
(i) sodium metal burns in limited supply of oxygen;
(ii) water is added to the product in (b)(i) above
(c) Describe a suitable laboratory procedure for comparing the conductance of 1 mol dm\(^{-3}\) aqueous solutions of sodium hydroxide and ethanoic acid.
(a)(i) What is meant by hydrocarbons?
(ii) A hydrocarbon consists of 92.3% carbon. If it’s vapour density is 39, determine its molecular formula. (H = 1; C = 12)
(b)(i) Outline a suitable laboratory procedure for obtaining ethanol from cassava tubers
(ii) List two laboratory reagents used for oxidizing ethanol to ethanoic acid.
(c) What name is given to each of the following processes?:
(i) Conversion of alkanols to alkanoates;
(ii) Breakdown of proteins to amino acids;
(iii) Conversion of oils to fats
(iv) Alkaline hydrolysis of fats and oils.
(a)(i) Draw a labelled diagram for the laboratory preparation of a dry sample of chlorine
(ii) Give one chemical test for chlorine.
(b) Write equations to represent the reaction of chlorine gas with: (i) iron (II) chloride solution;
(ii) potassium iodide solution;
(iii) hot concentrated sodium hydroxide solution.
(c) State what is observed on:
(i) bubbling hydrogen chloride gas into an solution of lead (II) trioxonitrate (V);
(ii) heating the mixture from (c)(i) above to boiling and aging it to cool.
(d) A solution of bismuth chloride was prepared by adding the oxychloride which is a white powder to concentrated hydrochloric acid. The following equilibrium was set up: BiOCI\(_{(s)}\) + 2HCI\(_{(aq)}\) \(\rightleftharpoons\) BiCl\(_{3(aq)}\) + H\(_2\)O\(_{(q)}\). State what would be observed if some water is added to the system. Explain your answer
Potassium trioxochlorate (V) undergoes thermal decomposition according to the fotpowing equation: 2KCIO\(_3\) \(\to\) 2KCI + 3O\(_2\)
(a) What substance could be used in the laboratory to in ease the rate of the reaction
(ii) absorb the oxygen produced
(b) Give the reason why an aqueous solution of silver trioxonitrate (V) gives a white precipitate with KCI but not with KClO\(_3\).
(a) State whether entropy increases or decreases during each of the following processes
(i) condensation of steam;
(ii) melting of wax;
(iii) dissolution of sugar in water;
(iv) abscas on charcoal.
(b) What deduction can be made in each case given that the value of the free energy change for a particular reaction is:
(i) zero;
ii) negative
When ethane – 1,2-dioic acid is heated with concentrated tetraoxosulphate (VI) acid, a reaction represented by the following equation occurs:
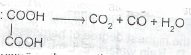
(a) State the type of process involved in the reaction
(b) What is the basicity of ethane-1, s-dioic acid?
(c) List three differences in the chemical properties of the two oxides of carbon produced during the reaction
(a) Write an equation to show the action of strong heat on:
(i) potassium trioxonitrate (V):
(ii) sucrose.
(b) State two observations in respect of the reaction between granulated zinc and dilute tetraoxosulphate (VI) acid.
(a) When MgSO\(_4\).XH\(_2\)O crystals were exposed to the atmosphere for several days, three was a loss in mass
(i) What name is given to this phenomenon?
(ii) Give another example of a compound that exhibits this phenomenon
(b) If 0.50 mole of MgSO\(_4\).XH\(_2\)O has a mass of 123g. calculate the value of X. (H = 1, O = 16, Mg = 24, S = 32)
(a) Define oxidation in terms of electron transfer.
(b) Consider the following: Cu\(_{(s)}\) + 2Ag\(^{+}_{(Ag)}\) \(\to\) Cu\(^{2+}_{(aq)}\) + 2Ag\(_{(s)}\)
(i) State the species that is reduced
(ii) Write half-cell equation for each of the species.

