(a)(i) Explain what is meant by an effective collision
(ii) What does each term represent in the following expression? \(\Delta G\) = \(\Delta H\) — T\(\Delta\)S
(iii) What deduction can be made about a system given that the value of its \(\Delta\)G is zero?
(b)(i) State Le Chatelier’s principle.
(ii) Suggest three conditions necessary for maximum yield of A\(_2\)B in the reaction represented by the following equation 2A\(_{2(g)}\) + B\(_{2(g)}\) \(\rightleftharpoons\) 2A\(_2\)B\(_{(g)}\) \(\Delta\)H = xkj.
(iii) Write an expression for the equilibrium constant of the reaction in (b)(ii) above.
(c)(i) Give the reason why a log of wood of a given mass does not burn out as quickly as sawdust of the same mass, under the same conditions.
(iii) List two examples of chemical reactions that are catalyzed by light
(a) Determine the oxidation number of manganese in MnO\(_4\)
(b)(i) List two metals that are extracted from their ores by electrolysis
(ii) Give one difference between a conductor and an electrolyte.
(a) State the monomer units of (i) polyethene; (ii) cellulose
(b) Name the two fuels obtained when steam and air are passed over red-hot coke.
(a) Give the name of the:
(i) process for obtaining ethanol from sugars;
(ii) compound produced when ethanol undergoes bacterial oxidation.
(iii) enzyme in yeast which catalyses the conversion of maltose to glucose.
(b)(i) Write the structural formula of ethanol
(ii) Name the organic product formed when propanoic acid reacts with ethanol.
Use the following energy profile diagram to answer Questions (a) to (c) below.
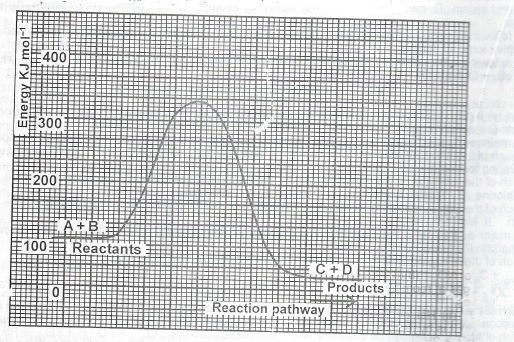
(a) From the diagram, determine the value of the; (i) enthalpy of the reaction (ii) activation energy of the reaction.
(b) State whether the profile diagram is for an endothermic reaction or an exothermic reaction.
(c) What name is given to substances which can provide an alternative pathway for the reaction?
(a) (i)Name the class of oxides to which copper (II) oxide belongs.
(ii) Give the reason why copper (II) oxide increases in mass on exposure to the atmosphere
(iii) Write an equation to show the action of dilute hydrochloric acid on copper (II) oxide.
(b) List two methods of removing total hardness from water.
(a) The elements listed below to the same group in the Periodic Table \(_9F\) \(_{17}CI\) \(_{35}Br\) \(_{53}I\).
(i) Write the electronic structure of the first member
(ii) What is the family name of the elements.
(iii) Which of the elements has the strongest oxidizing ability?
(b) Use the information provided in the following table to answer Questions (i) to (ii) below
(b)(i) Which of the chlorides would exist as discrete molecules?
(ii) What type of bonding holds atoms of A and chlorine together in ACI?
(iii) Which of the chlorides would be a good conductor of electricity?
(a)(i) State two physical properties of hydrogen sulphide
(ii) Name the laboratory equipment used for intermittent production of hydrogen sulphide
(b) What property of hydrogen sulphide is illustrated in the reaction represented by the following equation?
H\(_2\)S\(_{(g)}\) + 2NaOH\(_{(aq)}\) \(\to\) Na\(_2\)S\(_{(aq)}\) + H\(_2\)O\(_{(l)}\)
(a) What is the anhydride of each of the following acids?:
(i) H\(_2\)SO\(_4\)
(ii) HNO\(_3\)
(b) Classify each of the: following as normal salt/acid salt/basic salt/double salt
(i) Sodium hydrogentrioxocarbonate (IV)
(ii) Iron (III) chloride
(iii) Sodium ethanoate.
(a) State three postulates of the kinetic theory
(b) Name the process involved in the;
(i) spread of the smell of a perfume across a room
(ii) decrease in the yellow of a liquid after exposure in an open vessel.
(a) List two gases which are monatomic
(b) Mention one gas which forms:
(i) dense white fumes with ammonia vapour
(ii) yellow precipitate with ammoniacal silver trioxonitrate (V) solution.
(a)(i) List three characteristic properties of transition metals
(ii) 0.45g of a metal M was deposited when a current of 1.8 amperes was passed for 12.5 minutes through a solution containing M\(^{2+}\). Calculate the relative atomic mass of M. [1 Faraday = 96500 C]
(iii) Give the reason why copper-plated iron corrodes easily when the surface is scratched.
(b)(i) State the law of definite proportions (constant composition).
(ii) Describe in outline, an experimental procedure for determining the proportion of oxygen in a given sample of copper(II) oxide.
(iii) Write an equation to show how copper (II) oxide can be obtained directly from copper (II) trioxonitrate (V)
(a)(i) What is meant by cracking of petroleum fractions?
(ii) Write an equation for the laboratory preparation of ethene from ethanol.
(iii) Give one chemical test to distinguish between ethane and ethene.
(b)(i) Name the class of carbohydrates to which starch and cellulose belong.
(ii) What process is used for isolating ethanol from the other products of fermentation of sugar?
(iii) Name the organic product of the reaction between ethanol and sodium
(iv). Write the structural formula of 2-chloroethanol.
(c) State the reason why:
(i) benzene produces more soot than ethene on burning in excess air;
(ii) ethanoic acid has a higher boiling point than methanoic acid;
(iii) sodium chloride is used during the manufacture of soap.
(d) Give one use of: (i) ethyne (ii) coal (iii) carbon black
(a)(i) State two general methods of preparing soluble salts.
(ii) Mention three pieces of apparatus required for determining the solubility of a salt at a given temperature.
(b) The solubilities of two salts represented as K and L were determined at various temperatures. The results are shown in the table below:
| Temperature (\(^o\)C) | 0 | 20 | 40 | 60 | 80 | 90 |
| Solubility of K (mol. dm\(^{-3}\)) | 0.38 | 0.46 | 0.54 | 0.62 | 0.69 | 0.73 |
| Solubility of L (mol. dm\(^{-3}\)) | 0.12 | 0.34 | 0.64 | 1.08 | 1.64 | 2.00 |
(i) Plot the solubility curves of K and L on the same graph. Use the curves to answer questions (ii) – (iv) below.
(ii) What is the solubility of K at 50°C?
(iii) At what temperature is the solubility of L equal to 1.0mol. dm\(^{-3}\)?
(iv) Over what temperature range is K more soluble that L?
(v) Given that the molar mass of L is 101g, determine whether a solution containing 3.4g of L per 250cm\(^3\) at 20°C is saturated or unsaturated.
(a)(i) List two reactants for the laboratory preparation of ammonia
(ii) State three physical properties of ammonia
(iii) Describe in outline, the manufacture of ammonia by the Haber process.
(b) Write an equation in each case to show the:
(i) reaction between ammonia gas and heated copper (II) oxide
(ii) action of heat on ammonium trioxocarbonate (IV)
(c)(i) Which industrial process is used for convey ammonia to trioxonitrate (V) acid?
(ii) Give the reason why electropositive metals do not generally are off hydrogen with dilute trioxonitrate (V) acid
(d) Give one example in each case, to show how trioxonitrate (V) acid reacts generally with: (i) bases (ii) non-metals.
(a)(i) Define entropy
(ii) What term is used to describe a reaction in which heat is absorbed from the surrounding?
(b) State two conditions that can lead to ineffective collisions during a chemical reaction.
(a) Give one example of a metal which:
(i) can displace hydrogen from cold water
(ii) at red-heat, reacts reversibly with steam
(iii) does not react with dilute hydrochloric acid
(b) When dry hydrogen was passed over lead (II) oxide, a greyish solid J was obtained.
(i) Identify J
(ii) What type of reaction was involved in the formation of J from lead (II) oxide?
(a) Mention three chemical properties of chlorine
(b) What type of reaction is represented by each of the following equations?
(i) Mg\(_{(s)}\) + 2HCI\(_{(aq)}\) –> Mg\(_2\)Cl\(_{2(aq)}\) + H\(_{2(g)}\)
(ii) Ag\(^+_{aq}\) + Cl\(^-\)\(_{(aq)}\) —-> AgCl\(_{(s)}\)
(a) State Gay Lussac’s law of combining volumes
(b) Hydrogen reacts with oxygen according to the following equation;
2H\(_{2(g)}\) + O\(_{2(g)}\) —> 2H\(_2\)O\(_{(g)}\). If 50cm\(^3\) of hydrogen were sparked with 30cm\(^{3}\) of oxygen, calculate the volume of unused oxygen after cooling to the initial temperature and pressure.
(a) List two products obtained when crude oil is refined
(b)(i) What is the general formula for alkanols?
(ii) State the type of reaction involved in the conversion of ethanol to ethanoic acid
(iii) Write an equation to show how ethanoic acid reacts with sodium trioxocarbonate (IV).
Consider the reaction represented by the following Q equation: N\(_2\)O\(_{4(g)}\) \(\rightleftharpoons\) 2NO\(_{2(g)}\) \(\Delta\)H = +57.2KJmol\(^{-1}\)
(a) When is the reaction said to be at equilibrium?
(b) Mention two conditions that can favour the forward reaction
(c) Name the principle involved in (b) above.

