(a) The letters R,S,T represent an alkene, an alkene and a terminal alkyne respectively. Which of R, S and T typically undergo(es) the following reactions?
(i) Addition reaction;
(ii) Combustion;
(iii) Substitution reaction.
(b)(i) Name the process by which an alkanol can be converted to an alkene.
(ii) Write the name and structural formula of the third member cf the alkanol series.
(iii) State what would be obtained if a primary alkanol reacted with excess acidified KMnO\(_4\) solution.
(c)(i) Give one chemical test for alkanoic acids.
(ii) Write an equation to show how methanoic acid reacts with ethanol in the presence of mineral acids.
(iii) What is the role of mineral acid in the reaction in (c) ii?
(d) In an experiment, cassava was pressure-cooked to release starch granules, followed by treatment with malt for about 2 hours at 55°C. Yeast was then added and the mixture was left for 2 days at 27°C. An organic product J and a gas H were obtained.
(i) Identify H
(ii) State the class of carbohydrates to which starch belongs and explain what happened to the starch during treatment with malt.
(iii) Draw a labelled diagram of a suitable set-up for obtaining a sample of J from the reaction mixture.
a)(i) State three differences between covalent compounds and electrovalent compounds.
(ii) Two elements represented as K and L have atomic numbers of 12 and 8 respectively. Write their electronic structures and state the group to which each belongs in the Periodic Table.
(iii) If an alkali metal M exists naturally as the oxide, state with reason whether or not M can be extracted by reduction of the oxide with coke.
(b)(i) What is meant by the half-life of a radioactive element?
(ii) Mention the radioactive isotope used in dating archaeological specimens.
(iii) Balance the following equation and identify Q,
\(^{28}_{13}Al\) —> \(^{24}_{13}Si + \(^{0_1Q\)
(c)(i). Give three chemical properties of metals.
(ii) State two reasons why duralumin preferred to steel in aircraft manufacture.
(iii) What term is used to describe the ability of metals to be hammered into thin sheets?
(iv) Calculate the number of mole of electrons involved in the oxidation of 2.8g of iron filings to iron (II) ions. [Fe = 56].
(a)(i) State three postulates of the kinetic theory of gases.
(ii) Draw a sketch to illustrate Boyle’s law.
(iii) Explain what is meant by “absolute zero of temperature”.
(b) Equal volumes of a gas X and oxygen are at the same pressure but temperature of 200 K and 800K respectively.
(i) Giving reason for your answer, state which of the gas samples contains the greater number of molecules.
(ii) If the mass of one molecule of the gas X is 2.19 x 10\(^{-22}\) g, determine the molar mass of X. [Avogadro constant = 6.02 x 10\(^{-23}\)]
(iii) Hence, state with reason which of X and oxygen will diffuse faster under the same conditions. [O= 16].
(c)(i) Mention two reaction conditions that can increase the yield of ammonia in the reaction represented by the following equation: N\(_{2(g)}\) + 3H\(_{2(9)}\) \(\rightleftharpoons\) 2NH\(_{3(g)}\) \(\Dellta\)H = -46kJmol\(^{-1}\). Draw a labelled energy profile diagram for an endothermic reaction.
(iii) A solid W decomposes on heating according to the following equation: W\(_{(s)} \to Y_{(s}) + Z_{(g)}\) List two factors apart from temperature, which can affect the rate of the reaction.
(a) State how you would carry out the following procedures in the laboratory:
(i) Remove the sediment in sample of water;
(ii) Soften temporarily hard water without heating it,
(iii) Obtain pure water from muddy water;
(iv) Remove oxygen and moisture from a sample of air
(b)(i) What type of salts are alums?
(ii) State the function of alum in water treatment plants.
(iii) State and explain how rain water that hac passed through limestone deposits will react with soap solution.
(c)(i) Write an equation for the laboratory preparation of chlorine
(ii) List the products of the reaction of chlorine with hot concentrated sodium hydroxide solution
(iii) What is observed when moist blue litmus paper comes in contact with chlorine?
(iv) Calculate the volume of chlorine at s.t.p. that would be required to react completely with 3.70g of dry slaked lime according to the following equation:
Ca(OH)\(_{2(s)}\) + Cl\(_{2(g)}\) –> CaOCl\(_{2}\). H\(_2\)O\(_{(s)}\) [H = 1, O = 16, Ca = 40, 1 mole of gas occupies 22.4 dm\(^3\) at s.t.p.]
(d)(i) State what is observed on warming ammonium trioxonitrate (V) with sodium hydroxide solution
(ii) Explain why ammonium trioxocarbonate (IV) leaves no residue on being heated.
(a)(i) Give the names of two allotropes of sulphur.
(ii) State and explain what is observed when hydrogen sulphide is bubbled through acidified potassium tetraoxomanganate (VII) solution
(iii) List one product of the reaction of sulphur (IV) oxide with hydrogen sulphide
(b)(i) What are the raw materials for the manufacture of tetraoxosulphate (VI) acid by the contact process?
(ii) Write an equation for the reaction that requires a catalyst in the contact process and state the catalyst used.
(iii) State the observation and the product formed when concentrated H\(_2\)SO\(_4\) reacts with each of the following:
I. Copper turnings Ii. A cube of sugar
(c)(i) Give three uses of sodium trioxocarbonate (IV).
(ii) What name is given to reactions of the following type?:
exposure to air
Na\(_2\)CO\(_3\) + 10H\(_2\)O\(_{(s)}\) \(\to\) Na\(_2\)CO\(_3\); H\(_2\)O\(_{(S)}\) + 9H\(_2\)O
(iii) Calculate the solubility of Na\(_2\)CO\(_3\) at 25°C, if 20 cm\(^3\) of its saturated solution at that temperature gave 1.75g of the anhydrous salt. [C = 12, O = 16, Na = 23].
(a) What is meant by each of the following terms?:
(i) Esterification
(ii) Saponification
(b)(i) Give the general molecular formula of alkynes
(ii) Write the molecular formula and empirical formula of ethylethanoate.
(iii) Draw the structure of 1, 1, 2, 2-tetrabromoethane
(iv) Write an equation for the reaction of ethanol with sodium
(c) Consider the following reaction schemes:
I II
Petroleum —> Petroleum Fractions. Higher Petroleum Fractions —> Petrol + X

(i) State type of process/reaction involved in each of the stages labelled I to IV.
(ii) Identify X and Y
(iii) Give the IUPAC name of the product obtained in stage III.
(iv) What are the reaction conditions for stage IV?
(d) Explain why palm wine: (i) froths or foams (ii) tastes sour after some days.
(a)(i) List two properties of iron that are characteristic of transition metals
(ii) Using equations only, show the processes involved in the extraction of iron and the removal of impurities in the blast furnace.
(iii) The following reaction occurs when a piece of iron is exposed to moist air for some days:
4Fe\(_{(s)}\) + 3O\(_{2(g)}\) + xH\(_2\)O\(_{(l)}\) —> 2Fe\(_2\)O\(_3\)\(_{(s)}\) State three methods by which this reaction can be prevented
(iv) What is the oxidation number of iiron in the product in (iii) above?
(b)(i) Arrange the following metals in the order of increasing reactivity. Hence, state which of them is/are extracted by electrolysis Au, Zn, Mg, Na, Sn, Ca
(ii) Why is zinc said to be amphoteric?
(c)(i) Define oxidation in term of electron transfer
(ii) Determine how many moles of electrons are transferred when 4825 coulombs of electricity are passed through an electrolytic cell. [1F = 96500C]
(iii) Calculate the number of copper (II) ions that will be discharged by 0.250F. [Avogadro constant = 6.02 x 10\(^{23}\)
a)(i) State Graham’s law of diffusion.
(ii) Calculate the vapour density of a triatomic gas X if its relative: atomic mass is 16.
(iii) Equal volumes of gases Y and Z are maintained at the same temperature and pressure. If the mass of a molecule of Y is twice that of Z state and explain which of the molecules has the, greater average velocity.
(b) The graph below is the ratio curve for the following reaction carried out in an open vessel.
MgCO\(_{3(s)}\) + 2HCI\(_{(aq)}\) \(\to\) MgCl\(_{2(aq)}\) + CO\(_{2(g)}\) + H\(_2\)\(_{(l)}\).
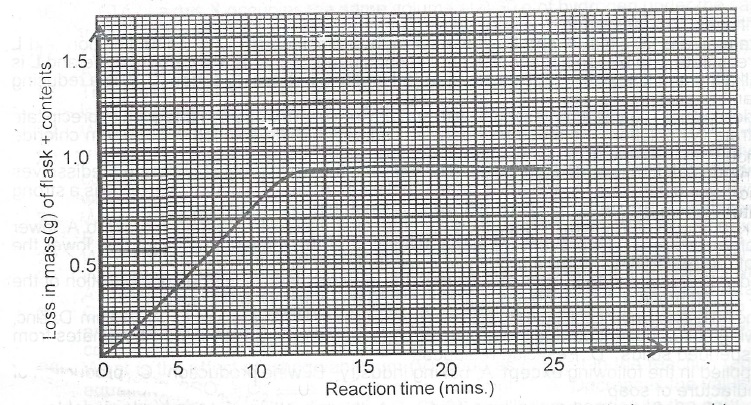
(i) For how long did reaction occur?
(ii) Why was there a loss in mass?
(iii) State whether reaction rate was fastest at the beginning, the middle or towards the end of the reaction. Give reason for our answer.
(iv) List three reaction conditions that can affect the slope of the curve
(c) Consider the following reaction at equilibrium: PCI\(_{5(g)}\) \(\rightleftharpoons\) PCI\(_{3(g)}\)); \(\Delta\)H = +95 kJmol\(^{-}\)
(i) Write an expression for the equilibrium constant K.
(ii) Predict the effect of the following on the equilibrium position.
I. Increased pressure
II. Increased temperature
III. Removal of chlorine Sketch an energy profile diagram for the forward reaction.
(a)(i) List three properties of elements which increase generally across a period in the Periodic Table,
(ii) Give two differences between a chemical reaction and a nuclear reaction.
(b) Use the information provided in the table below to answer Questions (i) – (vii).
|
Atom of Element |
P | Q | R | S | T |
|
Mass Number |
16 | 40 | 35 | 18 | 20 |
|
Atomic Number |
8 | 20 | 17 | 8 | 10 |
Which of the atoms in the table above:
(i) are isotopes of the same element?;
(ii) contains 18 neutrons?;
(iii) is chemically unreactive?;
(iv) readily forms an ion with two positive charges?
(v) attain an octet structure by accepting one electron?;
(vi) forms ionic bond with R?;
(vii) belongs to the s-block in the Periodic Table?
(c) Describe in outline how each of the following conversions can be carried out in the laboratory. Write appropriate equations for the reactions involved in each case
(i) CuCO\(_3\) to Cu
(ii) MgO to MgSO\(_4\).
(a) Describe briefly a suitable procedure for preparing a pure sample of MgSO\(_4\) starting from MgO.
(b)(i) Mention two sources of water pollution.
(ii) Explain why the sample of air collected in the process of boiling water is richer in oxygen than atmospheric air
(iii) Mention one substance used as coagulant in water treatment plants.
(c)(i) State two physical porperties of chlorine.
(ii) Write an equation to show how chlorine reacts with iron
(iii) Why is Chlorine preferred to sulphur (IV) oxide in the bleaching of cotton
(d) Bleaching powder reacts with dilute HCl according to the reaction below;
CaOCl\(_{2(s)}\) + 2HCI\(_{(aq)}\) -> CaCl\(_{2(aq)}\) + H\(_2\)O\(_{(l)}\) + Cl\(_{2(g)}\)
Calculate the mass of bleaching powder that will produce 400cm\(^3\) of chlorine at 25\(^o\)C and a pressure of 1.20 x 10\(^5\) NM\(^{-2}\). [O = 16.0; Cl = 35.5; Ca = 40.0;1 mole of gas occupies 22.4 dm\(^3\) at s.t.p; standard pressure = 1.01 x 10\(^6\) Nm\(^{-2}\)]
(a) List two substances that can be used in the laboratory to
(i) dry hydrogen;
(ii) remove carbon (IV) oxide from a sample of air;
(iii) convert hot copper (II) oxide to copper;
(iv) prepare zinc chloride by the action of dilute HCI.
(b)(i) Name two alloys which contain lead.
(ii) State and explain what is observed on bubbling H\(_2\)S into a solution of Pb(NO)\(_2\).
(iii) A metal M exists as a silvery white solid at temperatures above 18°C and as a grey solid below 18°C.
I. name the phenomenon exhibited by M.
II. What term is used to describe the temperature given as 18°C in this case?
(c)(i) Write an equation for the action of heat on each of the following compounds:
I. AgNO\(_3\)
II. (NH4)\(_2\)CO\(_3\).
(ii) Copy and complete the table below
|
Metal |
Name of main ore | Method of extraction |
One major use Haematite |
|
— |
Haematite | — |
— |
|
— |
— | Electrolysis of molten oxide |
— |
(a)(i) State three characteristics of a homologous series.
(ii) Give the name and structural formula of the second member of the alkyne series.
(iii) Write an equation to represent the combustion of ethane in excess oxygen.
(b) Name the type of reaction involved in the conversion of ethanol to
(i) ethene;
(ii) ethylethanoate;
(iii) chloroethane;
(iv) ethoxide
(v) ethanoic acid
(c) Consider the following compound.
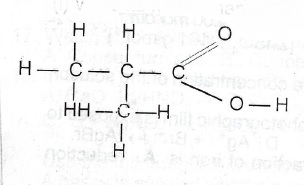
(i) Write its IUPAC name.
(ii) Give its molecular formula and empirical formula
(iii) List the products of its H H 0 reaction with saturated Na\(_2\)CO\(_3\) solution.
(iv) State with reason whether its boiling point will be higher or lower than that of the corresponding alkane.
(d) A vegetable oil X was treated with activated charcoal and then with a gas Y in the presence of a catalyst in order to manufacture
(i) Identify Y.
(ii) State the function of the activated charcoal.
(iii) What is the catalyst used?
(iv) If a sample of X is heated with concentrated sodium hydroxide solution, list the products that will be obtained.
(a)(i) Draw and label a simple cell for the electrolytic purification of copper.
(ii) Write can equation for the reaction at each electrode in (a)(i) above.
(iii) State with reason whether the Daniell cell is an electrolytic cell or an electrochemical cell.
(iv) What is the function of MnO\(_2\) in the Laclanche cell?
(b) Consider the following equation: MnO\(^-_4\) + 8H\(^+\) + xe\(^-\) \(\to\) Mn\(^{2+}\) + yH\(_2\)O. State the
(i) values of x and y;
(ii) oxidation state of Mn in MnO\(^-_4\).
(c)(i) List three factors that affect selective discharge of ions during electrolysis
(ii) State Faraday’s second law of electrolysis.
(iii) A voltameter containing silver trioxonitrate(V) solution was connected in series to another voltameter containing copper (II) tetraoxosulphate(VI) solution. When a current ri 0.200 ampere was passed through the solutions, 0.780g of silver was deposited. Calculate the
I. mass of copper that would be deposited in the copper voltameter
II. quantity of electricity used and the time of current flow. [Cu = 63.5 ; Ag = 108; 1F = 96500C]
(a)(i) Explain what is meant by acid anhydride and give one example
(ii) State three chemical properties of hydrochloric acid.
(b) Explain each of the following observations:
(i) Tetraoxosulphate (VI) acid can form two types of salts unlike trioxonitrate (V) acid.
(ii) Copper and iron react with concentrated H\(_2\)SO\(_4\) but only one of them reacts with the dilute acid.
(iii) On adding dilute H\(_2\)SO\(_4\) separately to zinc dust and zinc granules of the same mass, the dust produced more vigorous effervescence.
(c)(i) Define activation energy.
(ii) Sketch and label an energy profile diagram for the following reaction: A + B –> C + D; AH = xkJmol\(^{-1}\)
(iii) Explain why the heat of reaction of the mineral acids with sodium hydroxide is constant in value.
(d) Consider the reaction represented by the following equation:
Q\(_{(s)}\) \(\rightleftharpoons\) Q\(_{(l)}\) \(\Delta\) = xkJmol\(^{-1}\)
(i) State with reason which of Q\(_{(s)}\) and O\(_{(J)}\) has the higher entropy.
(ii) What will be the effect of decrease in temperature on the system at equilibrium?
(a) State three characteristic properties of
(i) electrovalent compounds;
(ii) alpha particles
(iii) catalysts
(b)(i) Write the electronic configuration of silicon (atomic number 14) and state the group to which it belongs in the Periodic Table.
(ii) State the type of chemical bonding between silicon and oxygen in SiO\(_2\)
(iii) A chip used in a microcomputer contains 5.72 x 10\(^{-3}\)g of silicon, calculate the number of silicon atoms in the chip.
[Si = 28; Avogadro constant = 6.02 x 10\(^{23}\) mol\(^{-1}\)]
(c) An element X belongs to the same group as sodium but is more reactive.
(i) Suggest with reason whether X would be a reducing or oxidizing agent.
(ii) What would be a suitable method of storing X in the laboratory?
(iii) Describe briefly what would be observed if a small piece of X were dropped into a trough of cold water which had been coloured with red litmus.
(iv) Write an equation to show how the oxide of X would react with dilute HCI.
(v) Suggest the likely colour of the salts of X
a)(i) Give two uses of ammonia.
(ii) Name the process by which ammoniacal liquor can be obtained from coal and list two other products of the reaction
(iii) What type of reaction is involved in the conversion of ammoniacal liquor to (NH\(_4\))\(_2\)SO\(_4\) by dilute H\(_2\)SO\(_4\)?
(iv) Sketch and label an energy profile diagram to show the effect of presence of Pt/Rh on the reaction represented by the following equation: 4NH\(_3\) + 5O\(_2\) \(\to\) 6H\(_2\)O + 4NO; \(\Delta\)H = —907 kJmol\(^1\)
(b) Rock salt is an impure form of sodium chloride.
(i) Outline a suitable procedure for preparing a pure sample of sodium chloride from rock salt.
(ii) State two methods that can be used to prepare chlorine from rock salt. Write an appropriate equation in each case.
(c) Lead pigments were used in a water colour painting which turned black after prolonged exposure to an air pollutant. The original colour was restored by using H\(_2\)O\(_2\) which converted the black substance to a simple, white lead (II) salt.
(i) Which pollutant turned the painting black?
(ii) Write the formula of the black substance
(iii) What is the white salt?
(iv) State the role of H\(_2\)O in the restoration process.
(a)(i) State three methods of preparing salts, giving one example in each case of a salt so prepared.
(ii) What type of salt is each of the following? NaH\(_2\)PO\(_4\); (CH\(_3\)COO)\(_2\)Pb; KAI(SO\(_4\))\(_2\). 12H\(_2\)O.
(b)(i) Write an equation for the reaction between dilute HCI and a solution of AgNO\(_3\).
(ii) Explain why NaNO\(_3\) is preferred to AgNO\(_3\) in the preparation of oxygen by thermal decomposition of trioxonitrate (V) salts.
(iii) When silver wire was dipped into an aqueous solution of CuSO\(_4\), the wire remained intact but when the wire was replaced with zinc rod, the rod decreased in size. Give an explanation for this observation.
(c) When a sample of a crystalline salt X was exposed to air, there was a loss in mass.
(i) What phenomenon was exhibited by X?
(ii) Suggest two substances which X could be.
(iii) On heating 5.00 g of a fresh sample of X to constant mass, 1.80g was lost in the form of water vapour. Calculate the number of molecules of water of crystallization in one molecule of X. [H = 1.00; O = 16.00; Anhydrous form of X = 160 g mol\(^{-1}\)
(a) Explain in terms of the kinetic theory why a tyre should not be overinflated.
(b)The following results were obtained at room temperature in an experiment to verify one of the gas laws using a glass syringe:
|
Pressure (P) of air in syringe (atm) |
Volume (V) of air in syringe (cm\(^3\) | \(\frac{I}{V}\) |
| 0.100 | 10.00 | 0.100 |
|
0.125 |
8.00 | 0.125 |
|
0.150 |
6.60 | 0.150 |
|
0.175 |
5.60 | 0.179 |
|
0.200 |
4.80 | 0.208 |
| 0.225 | 4.40 | 0.227 |
(i) Plot a graph of P against \(\frac{1}{v}\), using 1 cm to represent 0.01 atm on the vertical axis and 1cm to represent 0.02 unit on the horizontal axis.
(ii) Which of the gas laws is in agreement with the results?
(c) The flow chart below represents the stages involved in the manufacture of H\(_2\)SO\(_4\).
+x +Conc. H\(_2\)SO\(_4\) +H\(_2\)O
S + O\(_2\) \(\to\) SO\(_2\) \(\to\) SO\(_3\) \(\to\) Y \(\to\) Conc H\(_2\)SO\(_4\)
stage I stage II stage III stage IV
(i) Name the process represented by the chart.
(ii) Identify reactant X and product Y.
(iii) What are the operating temperature and pressure at stage II?
(iv) Mention the stage which requires a catalyst and state the catalyst used.
(v) Give the reason why the SO\(_3\) produced in stage II is not dissolved directly in water to form the acid
(d) When K\(_4\)Cr\(_2\)C\(_7\) dissolves in water, the following equilibrium is established:
Cr\(_2\)O\(^{2-}_{7(aq)}\) + H\(_2\)O\(_{(l)}\) \(\to\) 2CrO\(^{2-}_{4(aq)}\) + 2H\(_{aq}\)
(i) State the colour observed on adding a few drops of dilute H\(_2\)SO\(_4\) to the system.
(ii) Explain your answer in (d)(1).
(iii) What principle is applicable to this explanation?
(a) What term is used to describe each of the following processes?
(i) Alkaline hydrolysis of fats and oils;
(ii) The conversion of glucose into ethanol by enzymatic action;
(iii) Thermal decomposition of higher petroleum fractions into lower molecular mass hydrocarbons in the presence of catalyst.
(b)(i) Write the structure and IUPAC name for one alkanoic acid with the molecular formula C\(_4\)H\(_8\)0\(_2\).
(ii) Arrange the following compounds in order of increasing boiling point: Butane; Butanoic acid; Methylpropane.
(iii) Give an explanation for your answer in (b)(ii).
(c)(i) Ethanol was used for preparing a gas X which decolorized bromine water. Identify X and describe briefly its laboratory preparation.
(ii) Write an equation to show how ethanol reacts with sodium
(iii) Give the reagent and reaction conditions for the conversion of ethanol into C\(_2\)H\(_5\)COOC\(_2\)H\(_5\).
(d) State the type of recction involved in each of the conversions indicated below:
(i)C\(_6\)H\(_6\)C\(_6\)H\(_5\)CH\(_3\)
(ii) nC\(_2\)H\(_4\) \(\to\) (CH\(_2\) – CH\(_2\)),
(iii) CH\(_3\)CH\(_2\)CH(OH)CH\(_3\) -> CH\(_3\)CH\(_2\)CCH\(_3\)
(iv) (C\(_6\)H\(_{10}\)O\(_5\)) -> C\(_6\)H\(_{12}\)O\(_6\).
(iv)
(a) Giving different examples, mention one metal in each case which produces hydrogen on reacting with
(i) dilute mineral acid
(ii) cold water;
(iii) steam;
(iv) hot, concentrated alkali.
(b) In an experiment, excess 0.50 mol dm\(^{-3}\) HCI was added to 1Og of granulated zinc in a beaker. Other conditions remaining constant, state how the reaction rate would be affected in each case, if the experiment was repeated using:
(i) 1.0 mol dm\(^{-3}\) HCI;
(ii) 8.0g of granulated zinc;
(iii) 10g of zinc dust;
(iv) a higher volume of 0.50 mol dm HCI;
(v) a reaction vessel dipped in crushed ice;
(vi) equal volumes of water and 0.50 mol dm\(^3\) HCI.
(c) Aluminium is extracted from its ore by electrolysis.
(i) Name the ore from which the metal is extracted.
(ii) State the role of molten cryolite in the extraction.
(iii) Describe in outline how the ore is purified before electrolysis
(iv) Calculate the current in amperes required to produce 18.0g of aluminium in 1.50 hours. [Al = 27.0; F = 96500C]
(d) Give the reason why
(i) aluminium, which is a reactive metal, is resistant to corrosion.
(ii) metals are generally good reducing agents.
(a) What is the shape of (i) p – orbital; (ii) a molecule of methane; (iii) a molecule of carbon (IV) oxide?
(b) Consider the following elements: Ne, S, CI, 0, Fe, Mg. State which of them
(i) exhibit(s) allotropy;
(ii) form(s) coloured ions;
(iii) is/are malleable;
(iv) consist(s) of molecules that are far apart at room temperature;
(v) form(s) hydrides by sharing electrons with hydrogen;
(vi) has/have complete outermost shell.
(c)(i) List three applications of radioactivity in different fields.
(ii) Explain clearly the difference between the following reactions involving electron loss from lead.
\(^{211} pb\) \(\to\) \(^{ 211}Bi\) + \(^0_{-1}\); Pb \(\to\) pb\(^{3+}\) _ 2e\(^-\)
(iii) Give one advantage and one disadvantage of nuclear power generation over the use of fossil fuels.

